Monday April 15, 2024 Day 63 Thermodynamics of REDOX Reactions |
|
| Textbook
References 19.5: Cell Potential, Gibbs Energy, and the Equilibrium Constant P2: Table of Standard Reduction Potentials NOTE: Oxidation is ABOVE reduction in this table. |
Course Lectures 22.1 pdf Video* Thermo and Equil. connections |
| Standard Reduction
Potentials and Gibbs Free Energy Standard Reduction Potentials and Gibbs Free Energy |
Standard Cell
Potentials and Equilibrium Constants Standard Cell Potentials and Equilibrium Constants |
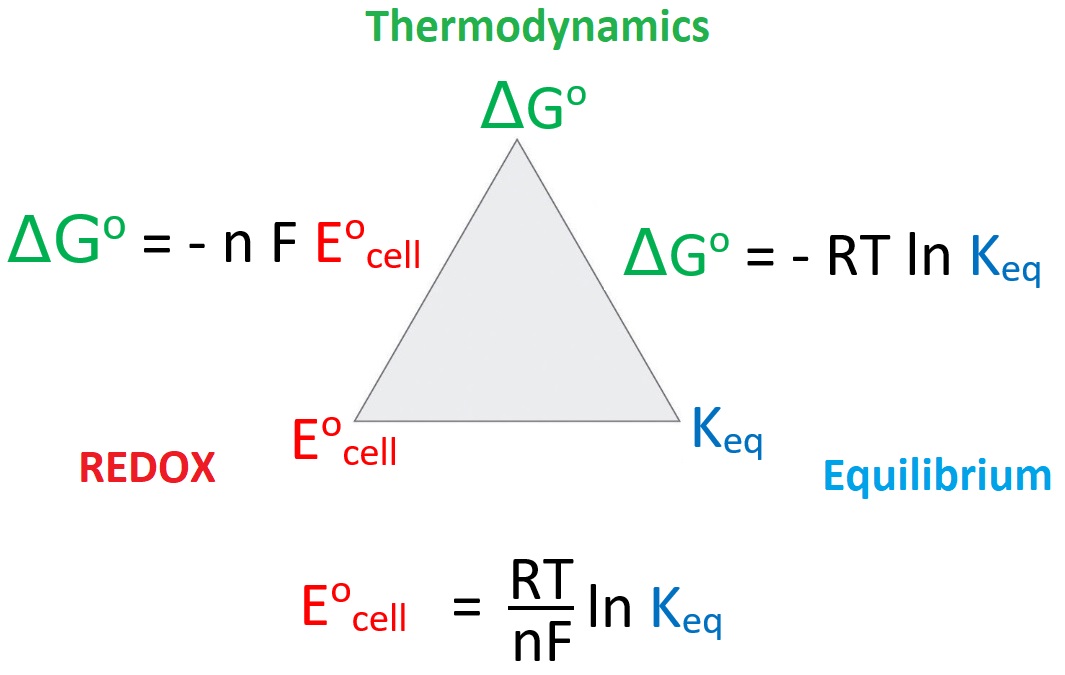
| Objectives 1. Utilize the REDOX, Equilibrium and thermodynamics relationships to determine ΔG°, Eocell and Keq from any one. 2. Use the signs and/or magnitudes of one parameter ( ΔG°, Eocell and Keq ) to make qualitative predictions about the remaining two. 3. Describe what must be true for ΔG°, Eocell and Keq in spontaneous and non-spontaneous situations. |
 |
| Homework Questions 56.1 Examine the following spontaneous REDOX reaction @ 25oC. 3 Zn(s) + 2 Cr3+(aq) → 3 Zn+2(aq) + 2 Cr(s) a. Determine: Eocell ΔG° rxn & Keq b. How are the values for consistant with a spontaneous reaction? 56.2 Examine this electrochemical cell @ 25oC: Zn| Zn2+(aq) (1.0 M) || Mg 2+(aq) (1.0 M) | Mg a. Write out the net cell reaction. b. Determine: Eocell ΔG° rxn & Keq c. Is the cell reaction spontaneous the way the abbreviated cell diagram is written? d. What are the characteristics Eocell , ΔG° and Keq under these circumstances? e. What changes are required (if any) to the abbreviated cell diagram? 56.3 The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, is given below. 2H2(g) + O2(g) → 2H2O(l) ΔG° = –474 kJ/mol What is are Eocell Keq for the fuel cell @ 25oC? 56.4 Consider the following information and use it to determine Eocell & ΔG° rxn @ 25oC. Zn(s) + Fe2+(aq) → Zn+2(aq) + Fe(s) K= 4.38 x 1010 Answers: Click and drag in the space below 56.1 a. Eocell = 0.0218 Volts ΔG° rxn = -12,620 J (n = 6) Keq = 162.6 b. All three quantities above are consistent with a spontaneous reaction: Eocell > 0 (positive) ΔG° rxn < 0 (negative) Keq >> 1 (HUGE) 56.2 a. Zn(s) + Mg2+(aq) → Zn2+(aq) + Mg(s) ...assumes cell diagram is correct with oxidation on the left. b. Eocell = -1.5942 Volts ΔG° rxn = + 3.076 x 105 J (n = 2) Keq = 1.19 x 10-54 c. non-spontaneous. First clue was that Mg was more towards the top of the reduction table and should've been the one oxidized. d. All three quantities above are consistent with a NON-spontaneous reaction: Eocell < 0 (negative) ΔG° rxn > 0 (positive) Keq << 1 (tiny) e. The diagram should be reversed: Mg | Mg 2+(aq) (1.0 M) || Zn2+(aq) (1.0 M) | Zn 56.3 Eocell = + 1.228 Volts Keq = 1.22 x 1083 56.4 Eocell = + 0.315 Volts G° rxn = -6.07 x 104 J |
|
Tuesday April 16, 2024 Day 64 The Nernst Equation: Non-Standard Cell Operation |
|
Text book References 19.6: Cell Potential and Concentrations P2: Table of Standard Reduction Potentials |
Course Lectures 22.2 pdf Video The Nernst Equation |
| Galvanic Cells:
Non-Standard Conditions (Nernst Equation)Galvanic
Cells: Non-Standard Conditions (Nernst Equation) |
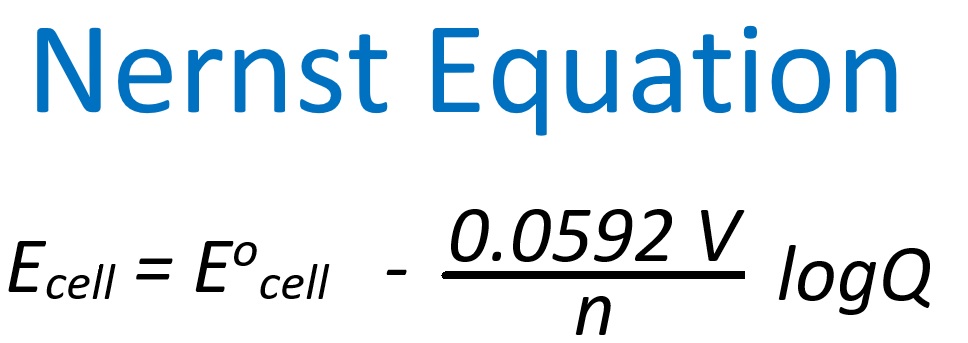 |
Objectives 1. Utilize the Nernst Equation to determine cell potentials under non-standard conditions. 2. Describe what happens to the cell potential as the cell operates converting reactants into products. 3. Determine cell ionic concentrations for different cell potentials |
|
| Homework Questions 57.1 Consider the following REDOX reaction and solution concentrations. What is the value of Eocell and Ecell ? Zn(s) + Cu2+ (aq) → Cu(s) + Zn2+ (aq) 1.00 M 0.0250 M 57.2 Consider the following REDOX reaction and solution concentrations. What is the value of Eocell and Ecell ? Zn(s) + Cu2+ (aq) → Cu(s) + Zn2+ (aq) 0.0250 M 1.00 M 57.3 Compare your Ecell values from 57.1 and 57.2. Which situation is more spontaneous and why? 57.4 Consider the following REDOX reaction and solution concentrations. What is the value of Eocell and Ecell ? 2Al(s) + 3Cd2+(aq) → 3Cd (s) + 2Al3+ (aq) 0.10 M 0.60 M 57.5 Consider the following voltaic cell. a. What is the net cell reaction? b. What is the value of Eocell and Ecell ? Cu(s) | Cu2+(aq) (0.25 M) || Ag+ (aq) (4.50 M) | Ag(s) c. What are the concentrations of Cu2+ and Ag+ when the cell potential has dropped to 0.43 Volts? |
|
| Answers: Click and
drag in
the space below 57.1 Eocell = 1.1037 V and Ecell = 1.1511 V 57.2 Eocell = 1.1037 V and Ecell = 1.0858 V 57.3 The more spontaneous cell has the larger Ecell value and this corresponds to problem 57.1 This makes sense as there's more reactant present in 57.1 and it makes sense that the reaction would shift right to make more products forcefully. (hence the larger Ecell value) This idea is actually at work as flashlight batteries wear out. As they're used, reactant is converted into product. Less reactant means smaller Ecell values and this lower voltage is responsible for the dimming of the light. 57.4 Eocell = 1.273 V and Ecell = 1.248 V 57.5 a. Cu(s) + 2 Ag+ (aq) (4.50 M) → Cu2+(aq) (0.25 M) + 2 Ag(s) b. Eocell = 0.4577 V and Ecell = 0.5141 V c. [Ag+] = 0.512 M [Cu2+] = 2.244 M Note that the silver ion concentration (a reactant) goes down and the copper ion concentration (a product) goes up as expected when the cell operates. |
|
Wednesday April 17, 2024 Day 65 The Nerst Equation: Concentration Cells |
|
| Text book References 19.6: Cell Potential and Concentrations |
Course Lectures 22.3 pdf Video Concentration Cells |
| Concentration Cells >Concentration Cells |
Concentration Cellos Concentration Cells |
| Objectives
1. Determine Ecell of a concentration cell 2. Predict concentration changes as the concentration cell discharges. 3. Relate concentration cell operation to entropy changes in the solutions. 4. Use the Nernst equation to determine the concentration of an unknown solution from the measured Ecell 5. Determine the pH of a solution using information from an H+ concentration cell. |
|
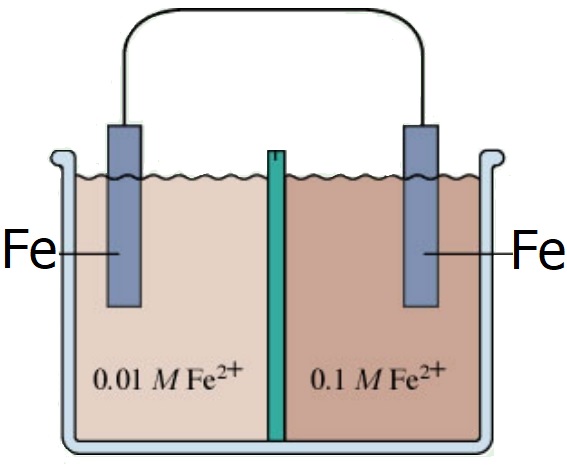
| Homework
Questions 58.1 Examine the concentration cell at right and... a. Write the ½ cell reactions making sure to include the solution concentrations in parenthesis. b. Write the net cell reaction again remembering to include the solution concentrations in parenthesis. c. Write the abbreviated cell diagram for this concentration cell. |
|
d. Determine Eocell, n and Ecell for this concentration cell e. Determine the direction of electron flow through the wire. f. Calculate the the Fe2+ molar concentrations when the cell no longer operates g. Qualitatively compare the entropy of the concentration cell initially and when discharged. 58.2 Examine the following concentration cell: Ni(s) | Ni2+(aq) (0.50 M) || Ni2+(aq) (3.00 M) | Ni(s) a. Write the net cell reaction including the solution concentrations in parenthesis. b. Determine Eocell, n and Ecell for this concentration cell c. What are the molar concentrations when the cell no longer operates? 58.3 Calculate the concentration of the unknown solution "x" for the following silver ion concentration cell: Ag(s) | Ag+(aq) (x M) || Ag+(aq) (1.00 M) | Ag(s) Ecell = 0.26 V 58.4 A pH probe is a H+(aq) concentration cell where the measured cell potential, Ecell, is used to determine an unknown hydrogen ion concentration and subsequently the pH. The following demonstrates the pH probe assembly using a standard hydrogen ½ cell (SHE) and another ½ cell containing an unknown H+ concentration. Determine the the unknown H+ concentration and the pH of the unknown solution: Pt(s) | H2(g) (1 atm) , H+(aq) (? M) || H+(aq) (1.00 M), H2(g) (1 atm) | Pt(s) Ecell = 0.45 V Click and drag below for answers 58.1 a. Oxidation Fe'(s) → Fe2+(aq) (0.01 M) + 2e- Note that this solution conc. is low and must increase. Reduction Fe2+(aq) (0.10 M) + 2e- → Fe''(s) Note that this solution conc. is high and must decrease. b. Fe'(s) + Fe2+(aq) (0.10 M) → Fe2+(aq) (0.01 M) + Fe''(s) c. Fe'(s) | Fe+2(aq) (0.01 M) || Fe+2(aq) (0.10 M) | Fe''(s) d. Eocell = 0 V n = 2 Ecell = 0.0296 V = 29.6 mV e. Electrons flow from left to right through the wire. f. Both solutions have a concentration of 0.055 M when the cell is completely discharged. g. Initially, there is lower entropy since one cell has high concentration and the other cell has low concentration. Entropy is greater when the cell is completely discharged and the ion concentrations are equal. In a sense, the ions have been distributed uniformly between the two half cells and we know from past experience that this is higher entropy. 58.2 a. Ni(s) + Ni2+(aq) (3.00 M) → Ni2+(aq) (0.50 M) + Ni(s) b. Eocell = 0 V n = 2 Ecell = 0.0230V = 23.0 mV c. Both solutions have a concentration of 1.75 M when the cell is completely discharged. 58.3 [Ag+]unk = 4.056 x 10-5 M 58.4 [H+]unk = 2.504 x 10-8 M pH = 7.601 Note: pH probes actually do contain 2 different ½ cells even though they appear to be a single assembly. One ½ cell, known as the "reference electrode" is housed in the probe assembly. This takes the place of the SHE electrode used above. The other ½ cell is created when the probe is placed in a solution containing hydrogen ions. Our Vernier data acquisition system measures the cell's Ecell and converts it into pH. |
|
Thursday April 18, 2024 Day 66 Common Cells and Batteries |
|
| Text book References 19.7: Batteries: Using Chemistry to Generate Electricity |
Course Lectures 23.2 pdf Video Common Battery Chemistries |
| Lead Acid
Batteries Lead Acid Batteries |
Fuel Cells Fuel Cells |
| Objectives 1. Define "cell" vs. "battery" 2. Qualitatively describe the 3 different battery chemistries at rightand their advantages and disadvantages. 3. Given Ecell, calculate the Ebattery for any number of cells. 4. Define the term "energy density" and how it applies to different battery chemistries. |
Lithium Ion Cells Lithium Ion Cells |
| Homework
Questions 59.1 In a lead-acid cell, during discharge the following reactions occur at the lead electrode surfaces: Pb(s) + HSO42–(aq) → PbSO4(s) + H+(aq) + 2e– PbO2(s) + HSO42–(aq) + 3H+(aq) + 2e– → PbSO4(s) + 2H2O(l) a. What is the net cell reaction? b. Use the Standard Reduction table to determin Eocell. c. A battery is made up of more than one cells in series. In a battery, all of the cells are connected "in series" ... meaning one-after-the-other. In this arrangement, each cell's Eocell value ADDS to the next. If car batteries are specified as 12 Volts, how many lead-acid cells are present? 59.2 In a fuel cell, during discharge the following reactions occur at the electrode surfaces: O2(g) + 4H+(aq) + 4e- → 2H2O(l) H2(g) → 2 H+(aq) + 2 e- a. What is the net cell reaction? b. Use the Standard Reduction table to determine Eocell. 59.3. In a lithium ion battery, the following net cell reaction takes place: LiC6(s) + CoO2(s) → C6(graphite) + LiCoO2(s) Eocell = 3.60 Volts a. Most cell phone batteries are rated at 3.60 Volts. How many cells are present? Is the term "battery" appropriate when talking about cell phones? b. If a rechargeable, cordless vacuum cleaner has a battery pack rated at 18.0 Volts, how many cells must be present in the pack? 59.4 Energy density is defined to be the amount of energy per liter. (Mega Joules per Liter) Specific energy is defined to be the amount of energy per kg (Mega Joules per kg) Consider the following: Gasoline: Energy Density = 34.2 MJ/L Specific Energy = 46.4 MJ/kg Li-ion Battery: Energy Density = 2.63 MJ/L Specific Energy = 0.875 MJ/kg a. If a fuel efficient automobile burns gasoline at a rate of 45 miles/gallon. How much energy in mega Joules is consumed during a 250. mile trip? Useful Info: Densitygasoline = 0.7489 g/cm3 1 gallon = 3.78541 L b. What weight of lithium ion batteries (kg and lbs) would be required to supply the same amount of energy? c. Based on the calculations above, it would seem that using lithium ion batteries instead of gasoline to move vehicles would require an unreasonable battery mass. However, electric motors are more efficient (~50%) than internal combustion gasoline burning engines (~30%) making more energy available for electrical motion. In light of this information, what weight of lithium batteries are now required for the 250 mile trip? 59.5 The hydrogen fuel cell converts hydrogen and oxygen gas into energy via the following Reaction: O2(g) + 2H2(g) → 2H2O(l) ΔGrxn = -474.2 kJ/mol a. How many moles of H2(g) would be required to produce the same amount of energy provided by gasoline in problem 59.4 a? b. Hybrid automobiles, like Honda's new Clarity (available in CA), use fuel cells and electric motors for propulsion. (see 59.4 c). Given the electric motor's high efficiency, how many moles of hydrogen gas would be required for the 250 mile road trip? c. Use the ideal gas law to determine the volume the H2(g) would require at T = 25oC and P = 1.0 atm. Is this a large volume of H2(g)? d. If all of the gas required (part b) were compressed into a steel pressurized gas tank (V = 50. L), what would the pressure of the gas be in atm and pounds per square inch? e. Do you see any problems associated with having compressed, high pressure hydrogen gas as an energy source in a fuel cell vehicle? Click and drag below for answers 59.1 a. Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l) b. Eocell = 2.046 volts c. Each cell produces approximately 2 volts. Thus it requires 6 cells to create 12 volts. 59.2 a. O2(g) + 2H2(g) → 2H2O(l) b. 1.229 volts 59.3 a. Only one battery is present in most cell phones. This would/should be more accurately referred to as "a cell" since there is only one present. b. There should be 5 cells in the battery pack. 59.4 a. 5.55 gallons of gasoline = 719.2279 MJ b. 822 kg = 1812 pounds of lithium ion batteries required. c. 493 kg = 1087 pounds for a 250 mile trip. 59.5 a. 1516.7 moles H2(g) b. 910.0 moles H2(g) c. Vol = 22264 liters (5882 gallons) of H2(g) at 1 atm d. 445 atm or 6543 psi H2 gas in a 50 L gas cylinder e. Oh yeah. "Filling up your car" means dispensing high pressure H2 gas. And how do we supply stations with a supply of H2 gas. Driving around in a vehicle with high pressure, compressed H2 could be dangerous. That said, proper engineering and safety protocols could make this a viable option to gasoline. The fact that the only biproduct is liquid water is desireable. However, electricity is required to make the H2 gas and if that electricity comes from fossil fuel burning electrical generation plants, the H2 produced is not "green." |
|
Friday April 19, 2024 Day 67 Electrolysis and Non-spontaneous REDOX Chemistry |
|
| Text book References 19.8: Electolysis: Driving Non-spontaneous Reactions with Electricity |
Course Lectures 23.1 pdf Video Electrolysis (non-spont REDOX) |
| Introduction to
Electrolysis Introduction to Electrolysis |
23.1 Electrolysis 23.1 Electrolysis |
| Homework
Questions 60.1 For batteries, the discharging process is spontaneous. As it happens, chemical potential energy (reactants) is convered into products and electrical energy that we use to power our phones, laptops, automobiles, etc. However, once a battery is exhausted, the energy must be replaced. This replacement procedure is known as charging and it doesn't happen on its own. Rather, an external energy source (a.k.a. the battery charger) pushes electrical current into the battery in the opposite direction This reverses the chemistry, reverses the REDOX reactions and converts products back into reactants at which point the battery is again charged. Battery charging is an example of electroysis: a process where non-spontaneous REDOX chemistry takes place with the help of an external energy supply. For the lead acid cell, the reverse reactions are: Charging- reduction: PbSO4(s) + H+(aq) + 2e– → Pb(s) + HSO42–(aq) Charging- oxidation: PbSO4(s) + 2H2O(l) → PbO2(s) + HSO42–(aq) + 3H+(aq) + 2e– Lead acid car batteries are often charged at 5.00 Ampere's (a.k.a. Amps or simply A) The Ampere is a measure of how much charge (in Coulombs a.k.a. C) that flows through the wire in 1 second. 1 Coulomb 1 Ampere(Amp) = ---------------- 1 second We also know the charge of an electron: 1.602 x 10-19 Coulombs or in other words: 1 electron = 1.602 x 10-19 Coulombs And, of course we also know that 1 moleelectrons = 6.023 x 1023 electrons a. Let's assume that a 5.00 amp current is used to charge a lead acid battery for 3.00 hours. Use dimensional analysis determine i. The charging current in units of C/s ii. The total charge (in Coulombs) that flowed during the 3.0 hours iii. The total number of electrons that flowed during the 3.0 hours iv. The moleselectrons of electrons that flowed during the 3.0 hours (Shortcut:
Faraday's constant, 96485.332 C/molee-
)
b. Refering now to the "Charging-reduction" ½ cell reaction above, we see that it takes 2 electrons to form 1 Pb atom. In other words there's a mole ratio that connects moles of electrons to moles of solid lead: 2 moleelectrons = 1 molePb Use this conversion factor to convert moles of electrons (part a iv) into moles of Pb. c. Next, determine the grams of Pb produced. Does this production of lead make the battery heavier? d. During this time, how many grams of water are converted into products? |
|
| 60.2 In the electrolysis cell shown
at right, an outside energy source is used to convert H2O(l) into H2(g) and O2(g) via the following two half reactions: 2 H2O(l) → O2(g) + 4 H+ (aq) + 4 e– 4 H+ (aq) + 4 e– → 2 H2(g) This process stores electrical energy as chemical potetntial energy: H2(g) and O2(g) These in turn can be used as fuel or an energy source in fuel cell applications. If the electricity comes from renewable resources (wind, solar), it would be considered "clean". |
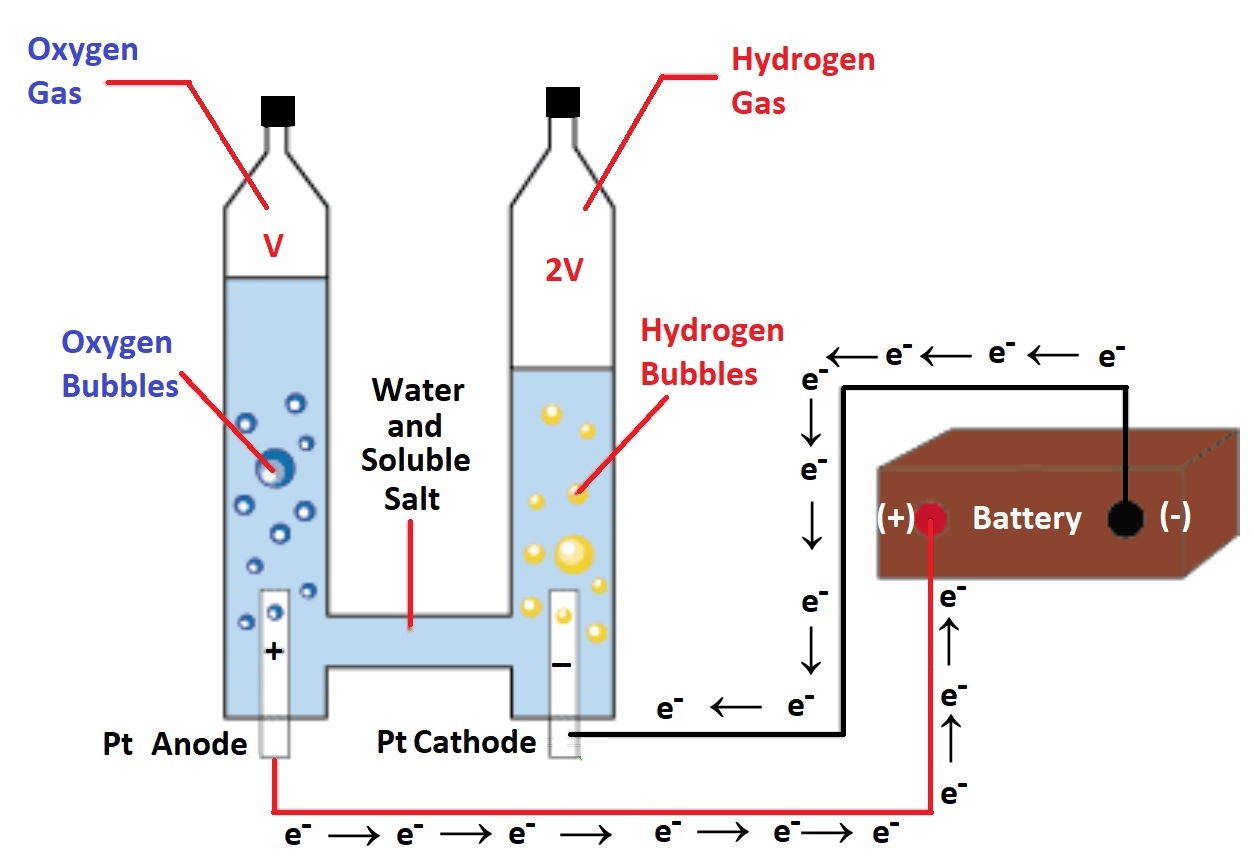 |
An electrical current 0f 0.450 Amps is passed through the electrolysis cell above for 10.0 minutes, what volume of oxygen and hydrogen gas in mL would we expect to collect? Assume 25.0oC and 1.00 atm pressure. 60.3 A 40.0 amp current flowed through molten iron(III) chloride for 10.0 hours. Determine the mass of iron and the volume of chlorine gas (measured at 25oC and 1 atm) that is produced during this time. Useful Information: anode (oxidation): 2 Cl- → Cl2(g) + 2 e- cathode (reduction): Fe3+ + 3 e- → Fe(s) |
|
| 60.4 Electrolysis has many
applications. For example, in the figure at right, two electrodes are immersed in a silver ion solution. The first electrode, is pure Ag and is where silver is oxidized & dissolves. The second electrode is a metal fork where silver ions in the solution are reduced and thus coat or "plate" the metal fork with a beautiful, shiny, Ag layer. Anode: Ag(s) → Ag+(aq) + 1 e- Cathode: Ag+(aq) + 1 e -→ Ag(s) A silver-plated fork typically contains about 2.00 g of Ag. If 12.0 h are required to achieve the desired thickness of the Ag coating, what is the average current that must flow during the electroplating process |
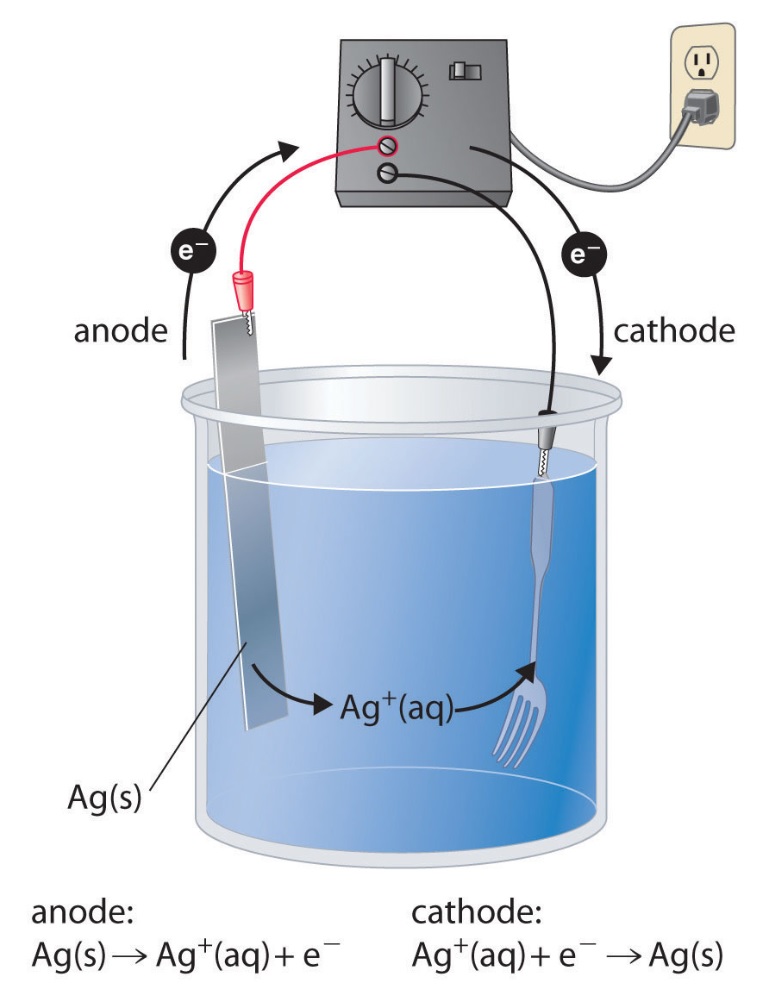 |
| Click
and drag below for answers 60.1 a. i. 5 C/s ii. 54000 C iii. 3.37079 x 1023 electrons iv. 0.559745 mol electrons b. 0.279873 mol Pb c. 58.0 grams Pb No. Conservation of mass requires the battery mass stay constant. d. 10.1 grams of water are converted into products 60.2 17.1 mL O2 and 34.2 mL H2 60.3 179 L of Cl2 gas and 278 grams of Fe(s) 60.4 Curent is 41.4 mA |
|