Monday January 22, 2024 Day 11 Factors Affecting the Rate of a Reaction |
|
| Text book References 3.3: Factors Affecting Reaction Rates |
Course Lectures |
| Objectives 1. Describe on a molecular level how each of the following increases the rate of a chemical reaction: a. Temperature b. Concentration c. Surface area/mixing d. Catalyst |
|
| Homework
Questions 61.1 On a molecular level, why does an increase in temperature increase the speed of a reaction? 61.2 On a molecular level, why does an increase in concentration increase the speed of a reaction? 61.3 On a molecular level, why does an increase in surface area increase the speed of a reaction? 61.4 On a molecular level, why does the presense of a catalyst increase the speed of a reaction? 61.5 In Principles of Chemistry 1, there is an experiment where students dissolve solid I2 in methanol before reacting the solution with solid Zn metal to form ZnI. You might remember it as the "Empirical Formula of ZnI experiement". One of the post-lab questions asks about the role that methanol plays and frequently students make the incorrect claim that it's a "catalyst". Why is that answer incorrect and what role really does the methanol play in increasing the rate of reaction? 61.6 In the video above, how do each of the following relate to reaction rate? a. Reducing hallway space b. Reducing time between classes c. The "match maker" d. Banning groups of students Answers: Click and drag in the space below 61.1 Increasing the temperature of a reaction mixture increases the molecular kinetic energies. Thus, when molecules collide, they will strike with greater force making chemical changes (bond breaking and rearrangement) more likely. 61.2 Increasing reactant concentrations (decreasing volume/additional solute or increasing pressure) makes things more crowded and increases the chance that molecules will collide. 61.3 By breaking up reactants, we expose them and increase the chance that transformative collisions will occur. Again: More collisions = Faster reactions. 61.4 Catalysts coordinate the reactants making sure they come into contact in just the right way and produce chemical change. 61.5 In this experiment, the methanol is the "solvent". It dissolves the I2 making it possble for more I2/Zn contact. The methanol really increases the surface area or mixing of the reactants. Methanol is NOT a catalyst as it does nothing to direct the correct orientation of reactants. 61.6 a. Less space = more crowding = higher concentrations = faster reaction b. Less time = faster students = more energetic collisions = faster reaction c. Match maker = orient students for successful encounters = a catalyst = more effective molecular collisions = faster reaction. d. No student clusters = more surface area = more collisions = faster reaction. |
|
Tuesday January 23, 2024 Day 12 The Rate of a Chemical Reaction: Reactants, Products and Coefficients |
|
| Textbook references 14.2 Rate of a Chemical Reaction |
Course Lectures 4.1 pdf Video* Reaction Rates |
| Objectives 1. Determine the rate of a chemical reaction from graphical information and the balanced chemical equation 2. Express the rate of a chemical reaction in terms of reactants or products given the balanced chemical equation |
|
| Homework
Questions 62.1 The graph at right was obtained for this reaction: 2A(g) → B(g). A tangent is drawn to the point high- lighted by the black dot. The slope of this line is represents the rate of change of "A" at that point. (a.k.a. the "instantaeous rate of change of A". What is the slope of the line and what are its units? |
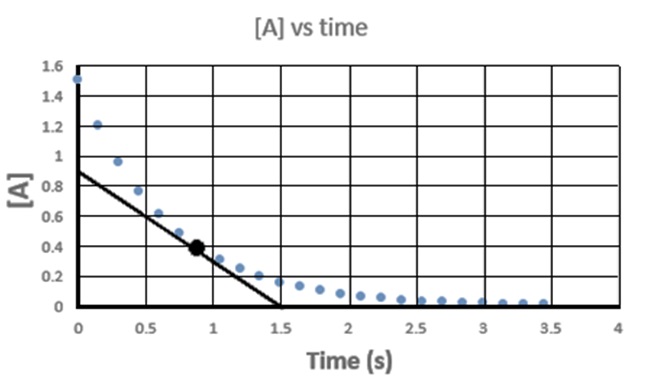 |
| 62.2
The slope of the line you determined in
62.1 is NOT the rate of this reaction for two reasons: i. The rate of the reaction is always reported as a positive number ii. The slope overestimates the rate since "A" is disappearing twice as fast as "B" is appearing. a. What is the rate of appearance of "B" in this case? b. What should be reported as the rate of this reaction? |
|
62.3 The graph at right was obtained for this reaction: 3A(g) + 2B(g) → C(g) + ½ D(g) Use the graph at right to determine the... a. The rates of change for all products and reactants. b. Rate of reaction |
 |
62.4 Consider the reaction N2(g) + 3 H2(g) → 2NH3(g) If the rate of formation of is measured to be NH3 is + 0.50 M/s, a. What are the rates of consumption for N2 and H2? b. What is the rate of reaction? 62.5 Consider the following reaction: 2 NO(g) + Cl2(g) → 2 NOCl(g) If Cl2(g) disappears at a rate of - 0.16 M/s, a. What is the production rate of NOCl? b. What is the rate of reaction? Answers: Click and drag in the space below 62.1 -0.60 M/s 62.2 a. + 0.30 M/s b. rate = 0.30 M/s (rate is always positive and independent of coefficients.) 62.3 a. RateA = - 1.018 x 10-3 M/s RateB = - 6.788 x 10-4 M/s RateC = + 3.394 x 10-4 M/s RateD = + 1.697 x 10-4 M/s b. Raterxn = 3.394 x 10-4 M/s 62.4 a. RateN2 = - 0.25 M/s RateH2 = - 0.75 M/s b. Raterxn = 0.25 M/s 62.5 RateNOCl2 = 0.32 M/s b. Raterxn = 0.16 M/s |
|
Wednesday January 24, 2024 Day 13 The Rate Equation |
|
| Textbook references 14.3 The Rate Law: The Effect of Concentration on Reaction rate |
Course Lectures 4.3 pdf Video The Reaction Rate Equation |
| |
|
| Objectives 1. Provide the general form of a reaction rate equation 2. Write the reaction rate equation given reactant order information 3. Identify reactant and overall reaction order given the reaction rate equation 4. Predict reaction rate changes given the reaction rate equation and concentration 5. From concentration and rate data, determine the reaction rate constant 6. Use the reaction rate law and rate constant value to determine the rate of the reaction. |
|
Homework Questions 63.1 A reaction is known to be first order in A, second order in B and zeroth order in C. a. Write the reaction rate equation b. How does the reaction rate change if the concentration of A is doubled and the other concentrations held constant? c. How does the reaction rate change if the concentration of B is doubled and the other concentrations held constant? d. How does the reaction rate change if the concentration of C is doubled and the other concentrations held constant? e. How does the reaction rate change if the concentration of all reactants are tripled? 63.2 Given the rate equation, rate = k [X][Y]2, answer the following questions. a. What are the individual and overall reaction orders? b. What are the units associated with all quantities in the rate equation? c. An experiment is performed where [X]i = 0.35 M [Y]i = 0.75 M If the rate of the reaction is measured to be 0.125 M/s, what is the value for "k" (with units)? d. Determine the rate of reaction when [X]i = 0.165 M [Y]i = 1.88 M (Assume conditions identical to part "c") 63.3 The following contains experimental data for the following reaction. The data summarizes experiments designed to determine the reaction rate equation for the reaction. NH4+(aq) + NO2-(aq) → N2(g) + 2H2O(l) Exp [NH4+]i [NO2-]i RATE ------------------------------------------------------- 1 0.010 M 0.020 M 0.020 M/s 2 0.015 M 0.020 M 0.030 M/s 3 0.010 M 0.010 M 0.005 M/s a. Examine the data highlighted in BLUE and answer the following questions: i. How does the concentration of [NH4+]i compare between the two trials? ii. How does the concentration of [NO2-]i compare between the two trials? iii. How has the reaction rate changed comparing the two trials iv. What does this say about the reaction rate order for [NH4+] ? Exp [NH4+]i [NO2-]i RATE ------------------------------------------------------- 1 0.010 M 0.020 M 0.020 M/s 2 0.015 M 0.020 M 0.030 M/s 3 0.010 M 0.010 M 0.005 M/s b. Examine the data highlighted in RED and answer the following questions: i. How does the concentration of [NH4+]i compare between the two trials? ii. How does the concentration of [NO2-]i compare between the two trials? iii. How has the reaction rate changed comparing the two trials iv. What does this say about the reaction rate order for [NO2-] ? c. Use what you've learned from parts a and b to write out the reaction rate equation. d. Use any experimental trial to determine the value of the rate constant k (with units) e. Determine the initial reaction rate when [NH4+]i = 0.018 M and [NO2-]i = 0.013M 63.4 Rate data were obtained for the following reaction: A + 2B → C + 2D EXP [A]i [ B]i RATE 1 0.10 M 0.10 M 3.0 x 10-4 M/min 2 0.30 M 0.30 M 9.0 x 10-4 M/min 3. 0.10 M 0.30 M 3.0 x 10-4 M/min 4 0.20 M 0.40 M 6.0 x 10-4 M/min a. Determine the rate law for this reaction b. Determine the value of the rate constant (with units) for this reaction c. Determine the initial reaction rate when [A]i = 0.25M and [B]i = 0.35M 63.5 The following data were obtained for the chemical reaction: A + B → C + D EXP [A]i [ B]i RATE 1 0.040 M 0.040 M 9.60 x 10-6 M/sec 2 0.080 M 0.040 M 1.92 x 10-5 M/sec 3. 0.080 M 0.020 M 9.60 x 10-6 M/sec Determine the initial reaction rate when [A]i = 0.060 M and [B]i = 0.030M Answers: Click and drag in the space below 63.1 a. rate = k[A]1[B]2[C]0 = k[A][B]2 b. 2 X rate c. 22 X rate = 4 X rate d. 20 X rate = 1 X rate e. 31 X 32 X 30 = 27 X rate 63.2 a. First order in X. Second order in Y. Third order overall b. [X]: Molarity1 [Y]: Molarity2 rate: M/sec k: M-2s-1 c. 0.6349 (1/M2 )(1/sec) d. 0.3703 M/s 63.3 a. i. A 1.5 X increase from trial 1 to 2 ii. No change iii. A 1.5 X increase from trial 1 to 2 iv. Since the rate depends directly on the concentration of NH4+ , it is first order in NH4+ . b. i. Concentration is the same. ii. Concentration doubles X 2 iii. Rate quadruples X 4 or X 22 iv. Since the rate depends upon the square of the NO2- concentration, it is second order in NO2-, c. rate = k [NH4+] [NO2-]2 d. 5000 M-2s-1 e. 0.01521 M/s 63.4 a. rate = k [A] (First order in A and zeroth order in B) b. k = 0.0030 min-1 c. 0.00075 M/min 63.5 Reaction is first order in A and B k = 0.0060 M-1s-1 rate = 1.08 x 10-5 M/s |
|
| |
|
Thursday January 25, 2024 Day 14 The Arrhenius equation Temperature and Activation Energy |
|
| Textbook references 14.5: The Effect of Temperature on Rxn Rate. |
Course Lectures 5.3 pdf Video Arrhenius Equation |
| Activation Energy |
Arrhenius Equation |
| Objectives 1. Describe how changing the reaction rate constant, kT, affects the rate of reaction. 2. Explain what activation energy is and how it affect the rate of reaction. 3. Describe how temperature affects molecular energies and how this relates to activation energy. |
|
| 4.
Explain how changes in temperature, activation energy, and frequency
affect the reaction rate constant, kT, determined via the Arrhenius Equation. 5. Given any three of the following variables: Temperature (T), Activation Energy (Ea), Frequency factor (A) or reaction rate constant (kT), determine the fourth using the Arrenius Equation. |
|
| Activation Energy: An anecdotal story Years ago, while out enjoying a motorcycle ride, I ran out of gas. The good news was that a gas station was near and at the bottom of a hill. The bad news was that I had to first push the bike up a hill before I'd be able to coast down to the gas station. It's a good thing I'd eaten breakfast because I'd need all my strength. The initial hill I'd have to overcome is known as "Activation Energy." |
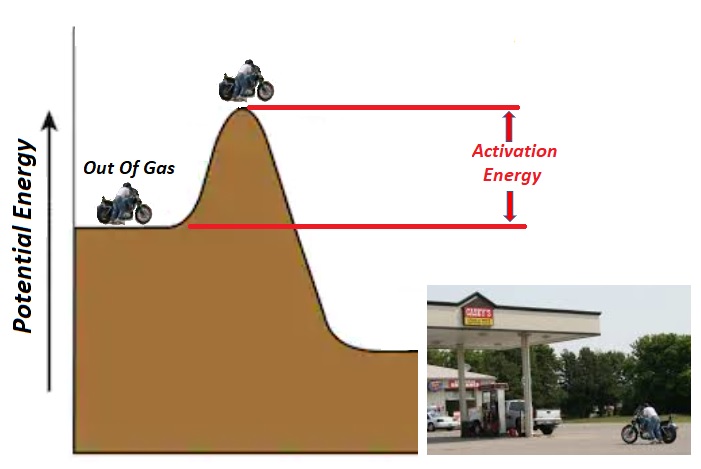
|
 |
Chemical reactions take place when molecules collide. In the figure at left, the reactants A & B must collide with enough energy to reach the top of the hill (Activated Complex) before beginning their downhill slide into products (C & D). The energy required to reach the top is known as the forward activation energy (Eaf). Molecules that collide with energies less than Eaf, will not go over the top and thus won't form products. |
| The kinetic energies of molecules depend on
temperature. As temperatures go up, so do molecular kinetic
energies. However, at a specific temperature, molecular kinetic energies are not a single value. Rather, they are distributed over a range of values. The figure at right demonstrates how this distribution changes as temperatures are increased T1 < T2 < T3 < T4 Notice that as temperature is increased, the distribution extends and flattens. So, at higher temperatures, a greater fraction of reactants have kinetic energies greater than the activation energy Eaf. and these reactants, if they collide, can form products. |
 |
| Recall that the rate of
a reaction
depends on the
concentrations of reactants and the reaction rate constant kT. rate = kT
[A]x[B]y[C]z
However, the rate constant kT depends on temperature and activation energy. This dependence is given by the Arrhenius equation:  |
|
| Homework
Questions 64.1 Refering to the "Molecular Fraction vs. Molecular Energy" graph above, what are the approximate percentages of molecules possessing energies greater than the activation energy at each of the four temperatures? 64.2 Given Ea = 50.0 kJ and A = 5.56 x 105. a. Use the Arrhenius calculate kT for 288 K and 298 K. b. How does kT change for this 10o C temperature increase? c. How does the reaction rate change for this 10o temperature increase? d. Chemists often say that the rate of reaction doubles for every 10o temperature increase. Is that the case here? 64.3 The decomposition of ozone an important reaction in atmospheric chemistry studies. O3(g) → O2(g) + O(g) Given the frequency factor (A) of 4.36 x 1011 and activation energy of 93.1 kJ/mol, Calculate the reaction rate constant at 25oC and 35oC . By what factor does the reaction rate increase in this example? 64.4 Consider the hydrolysis of sucrose shown below: HCl C12H22O11 + H2O → C6H12O6 + C6H12O6 Sucrose D-Glucose D-Fructose Given that Ea = 108 kJ/mol , kT = 1.0 x 10-3 M-1s-1 at 37oC a. Determine the frequency factor "A" that using the Arrhenius equation. b. Once known, you can assume that Ea and A don't change for a reaction. Use what you now know about the reaction above to determine the reaction rate constant kT at 100oC 64.5 The reaction rate constant for a reaction, kT is known to be 0.44 s-1 at 25oC If the forward activation energy is known to be 245. kJ/mol, what is kT at 125 oC? How many times faster does the reaction go at this elevated temperature? 64.6 An alternative form of the Arrhenius equation (2 point formulation) eliminates the frequency factor A and relates pairs of k and T values:  Given that kT = 0.75 s-1 @ 25oC and that kT = 11.5 s-1 @ 75oC, use the Arrhenius equation above to determine the activation energy for the reaction. 64.7 The two point Arrenius equation above can be used to create what's known as an Arrhenius plot. 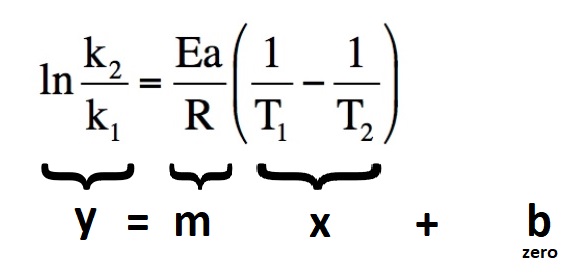 When (ln(k2/k1) is graphed versus (1/T1 - 1/T2), the straight line relationship let's us determine the activation energy from the slope: Consider the following Arrhenius plot constructed from the experimentally determined kT and temperature data. What is the activation energy for the reaction?  Click and drag below for correct answers. 64.1 The following percentages are visual approximations. Your results may vary somewhat. @ T1 (Orange) ~2% of all molecules have KE > Ea @ T2 (Orange + Red) ~ 20% of all molecules have KE > Ea @ T3 (Orange + Red + Green) ~ 30% of all molecules have KE > Ea @ T4 (Orange + Red + Green + Blue) ~45% of all molecules have KE > Ea 64.2 a. @ 288 K kT = 4.74 x 10-4 @ 298 K kT = 9.56 x 10-4 b. kT approximately doubles. c. Because the rate of reaction is proportional to kT, the reaction rate approximately doubles. d. Yes, while only an approximation, the reaction rate does seem to double for this 10o C temperature increase. 64.3 @ 298.15 K kT = 2.13 x 10-5 s-1 @ 308.15 K kT = 7.20 x 10-5s-1 3.4 X rate increase 64.4 a. A = 1.55 x 1015 b. @ 100oC kT = 1.18 64.5 @ 125oC kT = 2.66 x 1010 s-1 64.6 Ea = 47.12 kJ 64.7 Ea = 142.5 kJ |
|
End Week 3