Monday January 29, 2024 Day 15 Effective Collisions and Catalysts |
|
| Textbook references 14.5: The Effect of Temperature on Rxn Rate. 14.7: Catalysis |
Course Lecture 5.1 pdf Video Molecular Collisions 5.2 pdf Video Catalysts |
| Energy diagrams, Catalysts and Reaction
Mechanisms |
Catalyst Classes |
| Objectives 1. Identify forward and reverse activation energies from a reaction profile and use them to determine ΔHrxn and whether the reaction is exothermic or endothermic. 2. Inspect reactant and product molecular shapes and determine possible transition state (activated complex) arrangements. 3. Describe what is meant by an effective molecular collision and the factors that contribute to effective collisions. 4. Relate the magnitude of the frequency factor "A" in the Arrhenius equation to reactant collision orientations. 5. Describe what catalysts are and how they affect the rate of reaction on a molecular level. |
|
| Chemistry
takes place when atoms and molecules collide effectively. To be
an effective collision, the collision must satisfy two important
criteria: 1. The collisional energy must be greater than the activation energy Ea. 2. The reactant species must collide with the correct orientation to produce products. This last criterion is described by the frequency factor "A" in the Arrhenius equation:  |
|
As an example of a large "A" situation, consider the reaction below and at right: N(g) + O(g) → NO(g) Because both reactant atoms are roughly spherical (figure at right), it doesn't matter how they collide; only that they do. As long as their energies are greater than Ea, every collision is effective and produces products. In this case reactant orientation does not matter and A, kT and reaction rate are all larger. |
 |
As an example of a relatively smaller "A" situation, consider the following reaction: NO(g) + NO(g) → N2(g) + O2(g) Demonstrated below are two NO molecular collisions. To identify effective collisions from ineffective collisions, you must first look at the products to see what connections must be made. In this case we see that the two nitrogen atoms must connect with each other to form N2. Similarily, the two oxygen atoms must connect with each other to form O2. If, during the collision, the two N's and O's don't collide with eachother, the collision is ineffective. Referring to the diagram below, the first collision is ineffective as the N's and O's aren't lined up with each other when the molecules collide. In the second example, O's and N's contact each other forming what's called a transition state (also called an activated complex) which is positioned at the top of the activation energy hump. In the transition state, the N-N and O-O bonds are forming whilst the N-O bonds are breaking. When finished, the products go their separate ways. For this reaction, reactant molecule orientation does matter. Not all collisions have the right oreination to form products. Thus, A, kT and the reaction rate are smaller. |
|
 |
|
| Homework
Questions Referring to the figure at right .... 65.1 a. What is the value of the forward activation energy? b. What is the value of the reverse activation energy c. What is the value of ΔH for the reaction and is it exothermic or endothermic? |
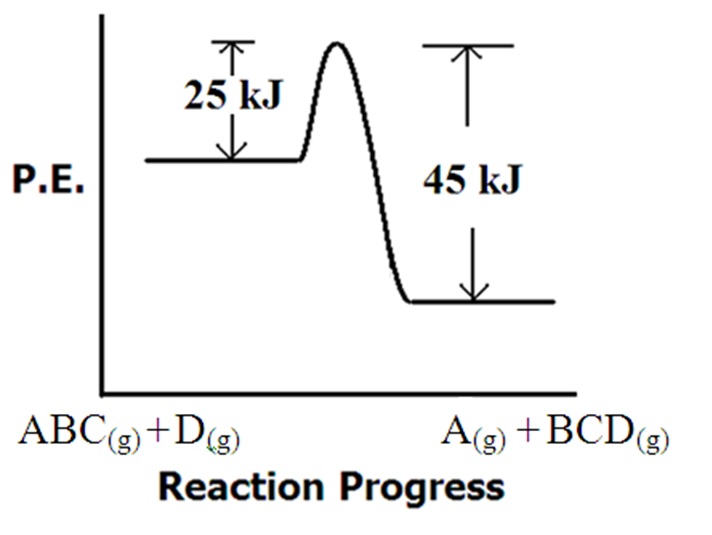
|
|
d. Will any collision between D and ABC produce a product?
(For help on this look at the products that form and decide how much they depend on collisional orientation) e. Which of the following is the most likely transition state for this process?  |
|
| 65.2
Reaction #1 has a very large frequency factor "A" while Reaction
#2 has a very small frequency factor comparatively. All other factors being equal, which of these two reactions should have a faster reaction rate? 65.3 Reaction A has a very small frequency factor value while Reaction B has a very large frequency factor value. All other factors being equal, which of these two reactions is less affected by molecular orientation? 65.4 Catalysts speed up chemical reactions. What variable changes in the Arrhenius equation when a catalyst is present? |
|
65.5 a. Referring to the figure at right, what is the value of ΔHrxn? b. What is the activation energy of the catalyzed reaction? c. By how much is the activation energy reduced when a catalyst is present? d. Why does the presense of a catalyst lower the forward activation energy? |
 |
| 65.6 As we'll learn later, chemical
reactions often occur in steps that together are considered a reaction mechanism. The introduction of a catalyst sometimes produces additional steps that occur behind the scenes which ultimately lower the activation energy and speed up the reaction. a. The figure at right shows the reaction profile both with and without catalyst. What is ΔH for the reaction? b. For the catalyzed reaction, how many steps take place behind the scenes? |
 |
|
c. What are the forward Ea values for each of
the catalysis steps? d. How much activation energy does the catalyzed reaction save in comparison to the uncatalyzed reaction? Answers: Click and drag in the space below 65.1 a. Eaf = 25 kJ b. Ear = 45 kJ c. ΔH = -20 kJ (exothermic) d. No. Refer to the reactants and products that appear along the x axis. Looking at the products, it's clear that "D" is attached to "C". Also, "A" is now separate. This will require bond formation between C & D and the A-B breaking. Consequently, when D collides with ABC, it'll be important for "D" to collide with the "C" end of the ABC molecule. e. "c" is the correct answer. It shows the A-B bond breaking and the C-D bond forming. 65.2 The rate of reaction is proportional to kT. kT is proportional to the frequency factor "A". Therefore, the rate of reaction is proportional to the frequency factor "A". All other factors being equal, the reaction with the larger frequency factor will be faster. 65.3 When molecules must collide in very specific ways, the frequency factor is smaller. When how the molecules collide isn't important, the frequency factor will be larger. Reaction B involves collisions that are less affected by molecular orientation. 65.4 Activation Energy Ea 65.5 a. + 12.5 kJ (endothermic) b. 32.3 kJ c. Activation energy lowered by 11.3 kJ d. Many reasons possible. The catalyst may introduce multiple steps to the process that have overall lower activation energies. Also the catalyst may direct the orientation of reactant species making the necessary bond breaking/making lower in energy. 65.6 a. - 56 kJ (exothermic) b. Two steps c. Ea1 = 26 kJ Ea2 = 21 kJ d. 32 kJ savings in activation energy. |
|
Tuesday January 30, 2024 Day 16 Integrated Rate Equations I |
|
| Textbook references 14.4 The integrated Rate Law: The Dep. of Concentration on Time |
Course Lectures 4.4 pdf Video Integrated Rate Equations 4.5 pdf Video Integrated Rate Equation Example |
| Objectives 1. Identify correct integrated rate expression to be used in a problem 2. Given initial concentration and rate constant, calculate the final concentration at some other time. 3. Given the concentration and rate constant, determine the initial concentration. 4. Adapt the integrated rate laws for use in situations utilizing percentages. |

|
The integrated rate law equations are powerful mathematical tools that let us determine what remains of a reactant after some amount of time has passed. These equations are listed in the table above as "Integrated Rate Laws". To use the equations, you must know the "order" of the reaction of which there are three: Zeroth "0", First "1" Second "2". Knowing this lets you determine which of the three equations to use. The remaining variables are: [A]0 ...the initial concentration or amount of "A" initially present. [A]t ... the amount of "A" present at time "t" k ... the reaction rate constant t ... time (any unit is okay as long as its consistent with "k") Knowning any 3 of these variables, lets you solve for the 4th. |
|
| Answer
these questions. 66.1 Zeroth order rate behaviors are frequently observed in manufacturing where product is constructed from a finite supply of parts. Consider the following overly simplified construction of automobiles: 4 Wheels + 1 Body → 1 Automobile a. Assuming unlimited "Bodies", how many automobiles could be constructed from the 500. wheels stored in the factory? b. The rate constant "k" for this zeroth order process is + 3.75 wheels/hr-1. How many hours will it take for the entire supply of wheels to be exhausted? c. Assuming an 8 hour workday, how many days will the wheel supply last? d. How many wheels are left after 3.5 work days? e. How many days are required to use 60. wheels? f. How many days are required to use half of the available supply of wheels? 66.2 A liquid evaporates following zero order kinetics with k = 6.75 x 10-3 g s-1. How long in seconds does it take for 0.010 kg of the liquid to evaporate? 66.3 The decomposition of SO2Cl2 is first order in SO2Cl2 and has a rate constant of 1.42 x 10-4 s-1. a. If the initial concentration of SO2Cl2 is 1.50 M, what is the concentration after 60.0 seconds have passed? b. How much time (s) is required for the concentration of SO2Cl2 to reach 0.50 M? c. How much time (s) is required to use 1.33 M of the original 1.50 M SO2Cl2 ? 66.4 Radioactive decay is a first order process. Consider Uranium 238 (U-238): It decays via first order kinetics with a rate constant of k = 1.551 x 10-10 yr-1. a. How much of a 10.0 gram sample is left after one billion years? b. How much of a 10.0 gram sample decayed after one billion years? c. How much time (yr) is required for 30% of the sample to remain? d. How much time (yr) is required for 30% of the sample to be consumed? 66.5 Carbon 14 (C-14) decays via first order kinetics with a rate constant of k = 1.2097 x 10-4 yr-1 . a. How many years have passed if 30.% of the original sample remains? b. How many years have passed if 99% of the original sample has decayed? Answers: Click and drag in the space below 66.1 a. 125 complete cars b. 133.3 hours c. 16.67 days d. 105 wheels used ... 395 wheels left e. 2 days f. 8.33 days 66.2 1,481 seconds 66.3 a. 1.487 M b. 7,740 seconds c. 1.53 x 104 sec. 66.4 a. 8.56 grams remaining b. 1.44 grams decayed c. 7.762 billion years d. 2.299 billion years 66.5 a. 9,953 years b. 38,000 years |
|
Wednesday January 31, 2024 Day 17 Integrated Rate Equations II |
|
| Textbook references 14.4: The Integrated Rate Law and how concentration depends on time |
Course Lectures 4.4 pdf Video Integrated Rate Equations 4.5 pdf Video Integrated Rate Equation Example |
| Objectives 1. Utilize graphical results to determine the reaction order. 2. Utilize graphical results to calculate the reaction rate constant. 3. Describe what is known as the "half life" for any physcial/chemical 4. Use half life formulations to determine half life and/or rxn rate constant. |
|
Integrated Rate Laws & Graphing The integrated rate laws can be graphed in ways to produce straight line behavior. At right, each of the three integrated rate law equations has been highlighted to identify the variables that should be graphed in search of a straight line plot. |
 |
| Typically,
experimental rate data (concentration and time) is graphed all three
ways: 0th order [A]t vs t 1st order ln[A]t vs t 2nd order 1/[A]t vs t The
graph that produces a straight line identifies the reaction order.
|
|
 |
|
| Answer
these questions 67.1 Refer to the graphs above and answer the following questions: a. In which graph does the data fit a straight line best? b. What is the reaction order in this example? c. What was the initial concentration of AB? i.e. [AB]0 d. What is the value of the reaction rate constant with units? e. Calculate the AB concentration at 230. seconds. f. Write out the reaction rate law for this reactant. 67.1 a. Third graph from left 1/[AB] vs time b. When the graph of 1/[AB] vs time is a straight line, the reaction is second order. c. 0.944 M d. 0.0225 M-1s-1 e. 0.160 M f. rate = k [AB]2 67.2 |
|
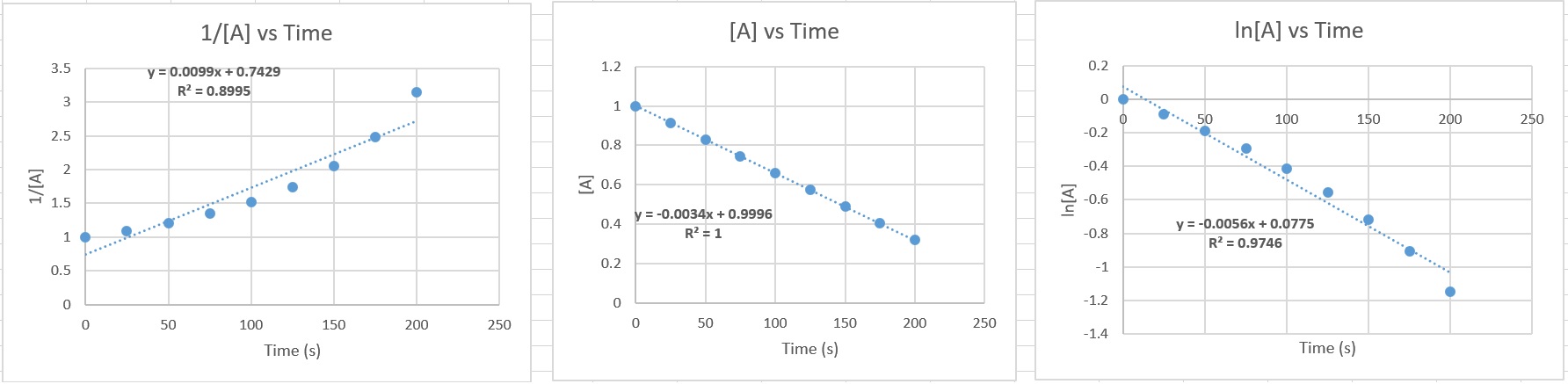 |
|
| 67.2 Refer to the graphs above and answer the following questions: a. In which graph does the data fit a straight line best? b. What is the reaction order in this example? c. What was the initial concentration of A? i.e. [A]0 d. What is the value of the reaction rate constant with units? e. Calculate the AB concentration at 250. seconds. f. Write out the reaction rate law for this reactant. 67.3 The half life, t½ , is the time required for exactly ½ of the original material to consumed. In the table above, the expressions are given for zeroth, first and second order reactions. Note that only the second order half life depends on the initial concentration. In all other cases, the half life is independent of concentration. a. The first order nuclear decay of U-238 has a rate constant of k = 1.551 x 10-10 yr-1 What is the half life for U-238 in years? b. The first order nuclear decay of carbon-14 has a rate constant of 1.2097 x 10-4 yr-1 What is the half life for C-14 in years? 67.4 Starting with 5 grams of a radioactive element (first order decay), how much remains after ..... a. 1 half life b. 2 half lives c. 3 half lives d. 4 half lives e. 10 half lives. 67.5 In the first order reaction D --> products, it is found that 90% of the original amount of reactant D decomposes in 140. minutes. Find the half life of the decomposition reaction. 67.6 The first-order decay of radon has a half-life of 3.823 days. How many grams of radon remain after 7.22 days if the sample initially weighs 250.0 grams? 67.7 Protactinium-231 has a half life of 3.24 x 104 years. How long will it take for 31% of the original sample to decay? Answers: Click and drag in the space below 67.1 a. Third graph from left 1/[AB] vs time b. When the graph of 1/[AB] vs time is a straight line, the reaction is second order. c. 0.944 M d. 0.0225 M-1s-1 e. 0.160 M f. rate = k [AB]2 67.2 a. Second graph from left [A] vs time b. Zeroth order c. 0.9996 M (~1.0 M) d. 0.0034 M/s e. 0.1496 M f. rate = k [A]0 = 0.0034 M/s [A]0 67.3 a. For U-238 t½ = 4.47 billion years b. For C-14 t½ = 5729.9 years 67.4 a. First half life = ½ x 5 grams = 2.5 grams b. Second half life = ½ x ½ x 5 grams = 1.25 grams c. Third half life = ½ x ½ x ½ x 5 grams = 0.625 grams d. Fourth half life = (½)4 x 5 gram = 0.3125 grams e. Tenth half life = (½)10 x 5 gram = 0.004883 grams 67.5 t½ = 42.14 minutes 67.6 67.5 grams remain .... 182.5 grams have decomposed. 67.7 1.73 x 104 years |
|
Thursday February 1, 2024 Day 18 Reaction Mechanisms I and the RATE LIMITING STEP (RLS) |
|
| Textbook references 14.6: Reaction Mechanisms Additional: Reaction Mechanisms |
Course Lectures 6.1 pdf Video Type 1 Rxn Mechanisms |
| The Rate Limiting Step |
The Rate Limiting Step |
| Objectives 1. Define the rate limiting step and identify rate limiting steps in real life 2. Identify the rate limiting step in type I reaction mechanisms 3. Define "elementary reaction" |
Type 1 Rxn Mechanisms |
4. Know what factors characterize an elementary reaction (uni or bimolecular) 5. Combine elementary reactions to determine the net reaction for the mechanism 6. Identify reaction intermediates in a reaction mechanism 7. Write the mechanism rate equation using the rate limiting step. |
|
| Answer
these questions 68.1 "Elementary reactions" are the individual steps that when taken together reproduce the overall reaction as the "net reaction." Why are uni-molecular and bi-molecular reactions allowable whereas tri-molecular reactions are not? 68.2 Examine the following two - step Type I reaction mechanism: Step 1 CH4(g) + Cl2(g) ⟶ CH3(g) + HCl(g) + Cl −(g) (Slow) Step 2 CH3(g) + Cl2(g) ⟶ CH3Cl(g) + Cl −(g) ( Fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? 68.3 Examine the following four-step Type I reaction mechanism: Step 1: HBr + O2 → HOOBr (Slow) Step 2: HOOBr + HBr → 2 HOBr (Fast ) Step 3: HOBr + HBr → H2O + Br2 (Fast ) Step 4: HOBr + HBr → H2O + Br2 (Fast ) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? 68.4 Examine the following four-step Type I reaction mechanism: Step 1: H2O2 → H2O + O (slow) Step 2: O + CF2Cl2 → ClO + CF2Cl (fast) Step 3: ClO + O3 → Cl + 2O2 (fast) Step 4: Cl + CF2Cl → CF2Cl2 (fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? 68.5 On the next exam, you'll be asked to describe a multi-step process from your life. You'll need to provide a list of the steps and then identify the RLS with reasons that explain why it's the rate limiting step. Answers: Click and drag in the space below 68.1 Tri-molecular reactions would require a collision between three atoms and/or molecules. The likelyhood of three particles colliding at exactly the same moment and with exactly the required orientation is low and therefore unlikely. 68.2 a. CH4(g) + 2Cl2(g) ⟶ CH3Cl(g) + HCl(g) + 2Cl −(g) b. CH3(g) c. Step 1 d. Rate = k[CH4][Cl2] 68.3 a. 4 HBr + O2 → 2 H2O + 2 Br2 b. HOOBr & HOBr c. Step 1 d. Rate = k[HBr][O2] 68.4 a. H2O2 + O3 → H2O + 2O2 b. O, ClO, CF2Cl, Cl c. Step 1 d. Rate = k[H2O2] 68.5 Looking forward to seeing what you come up with! :) |
|
Friday February 2, 2024 Day 19 Reaction Mechanisms II |
|
| Textbook references 14.6: Reaction Mechanisms |
Course Lectures 6.2 pdf Video Type 2 Reaction Mechanisms |
| Objectives 1. Define the rate limiting step and identify rate limiting steps in real life 2. Identify the rate limiting step in type II reaction mechanisms 3. Combine elementary reactions to determine the net reaction for the mechanism 4. Identify reaction intermediates in the type II reaction mechanism 5. Describe why the "slow" step that preceeds the RLS is an equilibrium reaction. 6. Use the equilibrium rate relationship to eliminate reaction intermediates in the reaction rate equation. |
|
Type II
reaction mechanisms
All reaction mechanisms contain steps that when combined produce a net reaction involving observable reactants and products. If one of the steps is slower than the others, it limits the speed with which the reaction can take place. The slowest step is referred to as the rate limiting step or RLS for short. In yesterday's material, the Type I reaction mechanism was introduced and today we'll look at the type mechanism. The two mechanism differ in one important way. While Type I mechanisms always begin with the rate limiting step, Type II mechanisms has the RLS following a fast equilibrium step.In other words, the RLS isn't the first step. It's important to understand why the equilibrium step that precedes the RLS happens at all. Consider the following simple Type II mechanism: Step 1 A ↔ B (fast) Step 2 B + C ⟶ D (Slow) Step 1 generates it's product B very quickly; the idea being that Step 2 can use B (& C) to make product (D). The problem is that Step 2 is very slow and consequently can't use B as quickly as Step 1 produces it. So, B levels begin to rise and as they do, the Step 1 reverse reaction becomes important. At that point, Step 1 experiences both forward and reverse reactions. This, as we know is when equilibrium exists. |
|
A Type II reaction mechanism's rate equation is derived much the same way as a Type I rate equation. However, an additional step is necessary to eliminate reaction intermediates from the final result. Consider the following Type II mechanism: Step 1 A + B ↔ C (fast) Step 2 C + D ⟶ E (slow) We immediately identify the RLS as Step 2 and use it to write down the rate equation: rate = k [C] [D] problem However, reaction rate equations must not include reaction intermediates and "C" is a reaction intermediate that must be eliminated. To do this, we refer to the "fast" equilibrium step that occurs before the RLS in the mechanism. As it is an equilibrium step, we know that the rates of the forward and reverse reaction are equal: rateforward = ratereverse We also know that i. rateforward = kf [A] [B] and ii. ratereverse = kr [C] Combining these relationships we have: kf [A] [B] = rateforward = ratereverse = kr [C] or more simply ... kf [A] [B] = kr [C] Now, solve for [C] (kf / kr) [A] [B] = [C] And substitute this into the rate equation derived from the RLS: rate = k [C] [D] rate = k (kf / kr) [A] [B] [D] Grouping the constants k, kf and kr all together as a new constant km (mechanism) we get our final result: rate = km [A] [B] [D] Note that this rate equation contains no reaction intermediates |
|
Answer these questions 1. Consider the reaction mechanism below: Step 1 H2 ↔ 2H (fast) Step 2 H + CO ⟶ HCO (slow) Step 3 H + HCO ⟶ H2CO (fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? e. What is the overall reaction order? 2. Consider the reaction mechanism below: Step 1 Cl2 ↔ 2 Cl (fast) Step 2 Cl + CHCl3 ⟶ HCl + CCl3 (slow) Step 3 CCl3 + Cl ⟶ CCl4 (fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? e. What is the overall reaction order? Answers: Click and drag in the space below 1. a. H2 + CO ⟶ H2CO b. HCO and H c. Step 2 d. Rate = k [CO] [H2]½ e. overall reaction order is 3/2 2. a. CHCl3 + Cl2 ⟶ CCl4 + HCl b. Cl and CCl3 c. Step 2 d. Rate = k [CHCl3] [Cl2]½ e. overall reaction order is 3/2 |
|
End Week 4