Monday February 19, 2024 Day 30 Bronstad Lowry Acids and Bases |
|
Textbook Readings 14.1 Bronstad Lowry Acids and Bases |
Course Lectures 10.1 pdf Video* Acid Base Models |
| Acid Base Models |
Arrhenius, Lowry-Bronstad and Lewis Acid - Base Models* |
| Objectives 1. Provide definitions for Arrhenius, Lowry-Bronstad and Lewis acids and bases. 2. Identify Arrhenius, Lowry-Bronstad and Lewis acids and bases individually and in chemical equations. 3. Identify conjugate acids and bases in chemical equations 4. Know the meaning of the term "amphoteric." |
|
| Homework Problems 21.1 Arrhenius acid/base model: What is an acid? What is a base? Lowry Bronstad acid/base model: What is an acid? What is a base? Lewis acid/base model: What is an acid? What is a base? 21.2 For each of the following reactions identify the reactant-side Lowry Bronstad acid and base. a. NH3(aq) + HCl(aq) ↔ NH4+(aq) + Cl-(aq) b. F-(aq) + H2O(l) ↔ HF(aq) + OH-(aq) c. H2O(l) + NH3(aq) ↔ NH4+(aq) + OH-(aq) d. H2CO3(aq) + OH-(aq) ↔ HCO3-(aq) + H2O(l) 21.3 For each of the reactions in 21.2 identify the product side Lowry Bronstad acid and base. 21.4 For each of the reactions in 21.2 record the conjugate acid base pairs. |
 |
21.5 Identify the acid base pairs in the reaction below. a. SO32-(aq) + H2O(l) ↔ HSO3-1(aq) + OH-(aq) b. HSO3-1(aq) + H2O(l) ↔ H2SO3aq) + OH-(aq) 21.6 A species that can behave both as an acid and a base is labeled "amphoteric." Examine your answers to 22.5 and determine the identity of the amphoteric species. 21.7 Water is amphoteric. Write an equilibrium reaction that has two water molecules as reactants and hydronium and hydroxide ions as products. 21.8 Identify the Lewis acid & base in each of the reactions above. Click and drag for answers below: 21.1 a. Arrhenius: Acid produces H3O+ and base produces OH- b. Lowry/Bronstad: Acid is a proton (H+) donor. Base is a proton (H+) receiver c. Lewis: Acid is an electron pair reciever. Base is an electron pair donor. 21.2 a. LB Acid: HCl LB Base: NH3 b. LB Acid: H2O LB Base: F- c. LB acid: H2O LB Base: NH3 d. LB acid: H2CO3 LB Base: OH- 21.3 For reverse reactions: a. LB acid: NH4+ LB base: Cl- b. LB acid: HF LB base: OH- c. LB acid: NH4+ LB base: OH- d. LB acid: H2O LB base: HCO3- 21.4 a. (acid...base) HCl ... Cl- & NH4+ ... NH3 b. (acid...base) H2O ...OH- & HF ... F- c. (acid...base) H2O ...OH- & NH4+ ... NH4+ d. (acid...base) H2CO3 ... HCO3- & H2O ...OH- 21.5 a. Base: SO32-(aq) Acid: HSO3-1(aq) & Acid: H2O(l) Base: OH-(aq) b. Base: HSO3-1(aq) Acid: H2SO3aq) & Acid: H2O(l) Base: OH-(aq) 21.6 HSO3-1(aq) is amphoteric as it can behave both as an acid or base depending on whether it is losing or gaining a proton (H+) 21.7 Acidic behavior H2O(l) → H+(aq) + OH-(aq) Basic behavior H2O(l) + H+(aq) → H3O+(aq) ------------------------------------------------------------------- Net Rxn: H2O(l) + H2O(l) → H3O+(aq) + OH-(aq) 21.8 Net Rxn: H2O(l) + H2O(l) → H3O+(aq) + OH-(aq) (acid) (base) (acid) (base) |
|
Tuesday February 20, 2024 Day 31 The Relative Strengths of Acids and Bases |
|
Textbook Readings 14.3: The Relative Strengths of Acids and Bases. 16.4: Acid Strength & Ka |
Course Lectures 10.2 pdf Video* Strong/Weak Acid Comp. |
| Memorize The Strong & Weak Acids &
Bases |
8.3 Strong and Weak Acids and Bases |
| Objectives 1. Describe what is meant by a strong acid, weak acid, strong base and weak base. 2. Predict the conjugate base strength for corresponding strong or weak acids. Predict the conjugate acid strength for corresponding strong or weak bases. 3. In an acid/base equilibrium, identify the strong acid, weak acid, strong base and weak base. 4. Predict the magnitude of the acid dissociation constant given relative product and reactant amounts. 5. Identify strong and weak acids based on a comparison of equilibrium constants; i.e. Ka values .....Identify weak/strong acids, bases, conjugate acids and conjugate bases. .....Suggest Ka values for acids based upon their strength. ..... Compare Ka values and use to list the acids according to their strengths. .....Use Ka value to order conjugate bases according to their strengths. |
|
| Homework Problems 22.1 Strong acids undergo complete ionization in water. This reaction only makes products: HA(aq) + H2O(l) → H3O+(aq) + A-(aq) Use the table at right to write the strong acid ionization reactions for the 5 "strong" acids. These strong acids should be memorized. 22.2 Weak acids undergo partial ionization in water. This equilibrium reaction has both reactants and products present in equilibrium: HA(aq) + H2O(l) ↔ H3O+(aq) + A-(aq) |
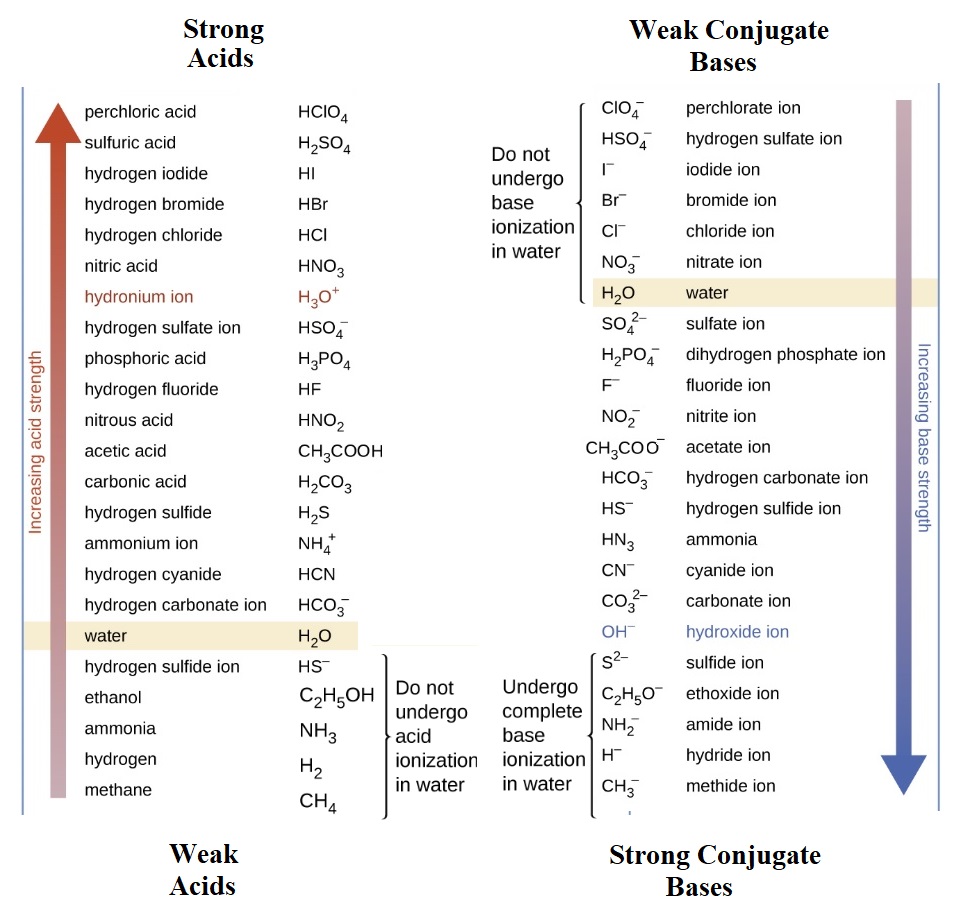 |
Use the table above to write weak acid dissociation equilibrium reactions for a. hydrofluoric acid b. nitrous acid c. carbonic acid 22.3 Strong bases undergo a complete reaction with water that forms hydroxide ions. This reaction only makes products: B-(aq) + H2O(l) → OH- (aq) + BH(aq) Notice that the strong base, B-(aq) , isn't actually present once the reaction occurs but is instead found as BH. Use the table above to write the strong base reaction for the following strong bases. a. methide b. amide c. sulfide 22.4 Weak bases undergo a partial reaction with water that forms small numbers of hydroxide ions in equilibrium: B-(aq) + H2O(l) ↔ OH- (aq) + BH(aq) Note that the 7 weakest bases in the table above are so weak that a reaction with water doesn't occur. Write the weak base equilibrium reactions for the following weak bases a. chloride b. perchlorate c. fluoride d. ammonia 22.5 How are the chemical equations that describe strong and weak acids with water the same/different? How are the chemical equations that describe strong and weak bases with water the same/different? 22.6 What distinguishes a strong acid from a weak acid? What distinguishes a strong base from a weak base? 22.7 Consider the equilibrium reaction of acetic acid in water:  The small equilibrium constant (1.76 x 10-5) tells us that reactants are favored. The bar graphs below each species represents an equilibrium concentration and are consistent with a small equilibrium constant. H2O isn't shown as a bar graph since it is a liquid and not included in the Law of Mass Action. a. Of the two species labeled "acid", which is present in a greater amount? Label this species "WEAK ACID" as it does not successfully dissociate. b. Of the two species labeled "acid", which is present in a lesser amount? Label this species "STRONG ACID" as it more successfully dissociates. c. Of the two species labeled "base", which is present in a greater amount? Label this species "WEAK BASE" as it doesn't successfully acquire an H+ ion. d. Of the two species labeled "base", which is present in a lesser amount? Label this species "STRONG BASE" as it does successfully acquire an H+ ion. 22.8 Consider the following reaction and its large equilibrium constant. F-(aq) + H3O+(aq) ↔ HF(aq) + H2O(l) K = 1.4 x 103 Identify all acid base conjugate pairs and label them as strong or weak. Click and drag for answers below. 22.1 Six of them! HCl(aq) + H2O(l) ⟶ H3O+(aq) + Cl-(aq) HNO3(aq) + H2O(l) ⟶ H3O+(aq) + NO3-(aq) HBr(aq) + H2O(l) ⟶ H3O+(aq) + Br-(aq) HI(aq) + H2O(l) ⟶ H3O+(aq) + I-(aq) H2SO4(aq) + H2O(l) ⟶ H3O+(aq) + HSO4-(aq) HClO4(aq) + H2O(l) ⟶ H3O+(aq) + ClO4-(aq) 22.2 a. HF(aq) + H2O(l) ↔ H3O+(aq) + F-(aq) b. HNO2(aq) + H2O(l) ↔ H3O+(aq) + NO2-(aq) c. H2CO3(aq) + H2O(l) ↔ H3O+(aq) + HCO3-(aq) 22.3 a. CH3-(aq) + H2O(l) → OH- (aq) + CH4(aq) b. NH2-(aq) + H2O(l) → OH- (aq) + NH3(aq) c. S2-(aq) + H2O(l) → OH- (aq) + S2H(aq) 22.4 NOTE: "a" and "b" are such weak bases that even though we can write their equilibrium reactions, we understand that virtually no products are ever present. a. Cl-(aq) + H2O(l) ↔ OH- (aq) + HCl(aq) b. ClO4-(aq) + H2O(l) ↔ OH- (aq) + HClO4(aq) c. F-(aq) + H2O(l) ↔ OH- (aq) + HF(aq) d. NH3(aq) + H2O(l) ↔ OH- (aq) + NH4+(aq) 22.5 a. All acid reactions have water as a reactant and hydronium (H3O+) as a product. However, strong acids are considered completion reactions as for all practical purposes only hydronium and the weak base ion are present. Weak acids are in equilibrium with their dissociation products and all are present in real amounts. b. Like acid dissociation reactions, base reactions also have water as a reactant but instead have hydroxide (OH-) as a product.. Strong bases produce hydroxide completely while weak bases produce hydronium ions at lower levels in an equilibrium situation. 22.6 Strong acids produce 100% hydronium ions. Weak acids produce far fewer and there's an equilibrium situation with water as a reactant. Strong bases produce 100% hydroxide ions. Weak bases broduce far fewer and there's an equilibrium situation with water as a reactant. 22.7 a. Comparing the two acids, H3O+ and CH3COOH, the latter is present in higher concentrations and is therefore the weaker acid. b. Comparing the two acids, H3O+ and CH3COOH, H3O+ is present in lower concentration and is therefore the stronger acid. c. Weak bases are created by strong acids and since the strong acid is H3O+, the weak base is H2O. d. By similar reasoning, the strong base is always associated with the weak acid. Therefore CH3COO- is the stronger base. 22.8 F-(aq) + H3O+(aq) ↔ HF(aq) + H2O(l) K = 1.4 x 103 In this case, the equilibrium constant is very large and so we know that there's a lot more product present than reactant. This let's us compare acid and base levels on either side of the arrow to determine their relative strengths. HF Weaker acid (Higher conc) .... associated with stronger base F- H3O+ Stronger acid (lower conc) .... associated with weaker base H2O |
|
Wednesday February 21, 2024 Day 32 Autoionization of Water: pH and pOH |
|
Textbook Readings LT: 16.5 Autoionization of Water and pH |
Course Lectures 10.3 pdf Video* Conj. Acid/Base and Recipes |
| Objectives 1. Write the equilibrium reaction for the autoionization of water 2. Write the Law of Mass Action for the autoionization of water 3. Memorize the value of Kw at 25oC 4. Given any two of three variables, calculate the third: [H3O+] [OH-] = Kw 5. Qualitatively know how the autoionization reaction shifts knowing it's endothermic . |
|
| 6. Calculate pH, pOH and pKw: pH = - log [H3O+] pOH = - log[OH-] pH + pOH = pKw = 14 @ 25oC pKw = -log Kw |
|
Homework Problems 23.1 What is the chemical reaction that describes the autoionization of water and why is water considered amphiprotic? 23.2 All of the equilibria we study are in aqueous environments. Because water is present in very large amounts, its autoionization reaction establishes the connection between [OH-] and [H3O+] via the Law of Mass Action. What is this mathematical relationship? 23.3 Kw is the equilibrium constant for the autoionization of water. At 25 oC, Kw = 1.00 x 10-14. What are [H3O+] & [OH-] for distilled water at 25 oC? 23.4 At 30 oC, Kw = 1.47 x 10-14. What are [H3O+] & [OH-] for distilled water at 30 oC? 23.5 What are the pH and pOH values for problems 23.3 and 23.4? 23.6 Experimentally, we'll measure the pH of a solution using a pH probe. From the pH, we can determine the molar concentration of [H3O+] & [OH-] a. Start with pH = - log [H3O+] and derive a relationship that lets us calculate [H3O+] from pH. b. What are the [H3O+] & [OH-] concentrations if the measured pH = 3.56? 23.7 As you can see from problems 23.3 and 23.4, Kw increases with increasing temperature. Is the autoionization of water an exothermic or endothermic reaction? Explain. Answers: Click and drag in the space below 23.1 H2O(l) + H2O(l) ↔ H3O+(aq) + OH-(aq) Water is amphoteric since it can behave as either a Lowry Bronstad acid OR base depending on the situations. In the autoionization reaction, one water molecule behaves as an acid and the other as a base. 23.2 Kw = [H3O+] [OH-] 23.3 [H3O+] = [OH-] = 1.00 x 10-7 M 23.4 [H3O+] = [OH-] = 1.21 x 10-7 M 23.5 For 23.3 pH = pOH = 7.00 For 23.4 pH = pOH = 6.91 23.6 a. [H3O+] 10-pH b. [H3O+] = 2.75 x 10-4 M [OH-] = 3.63 x 10-11 M 23.7 Increasing the temperature increases the value of the equilibrium constant Kw. A larger equilibrium constant is consistent with more product and less reactant. Therefore, "heat" is a reactant (endothermic). Adding heat, a reactant shifts the reaction right. |
|
.
Thursday February 22, 2024 Day 33 Weak Acid Equilibrium |
|
Textbook Readings 16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions |
Course Lectures 11.1 pdf Video* Weak Acid Equilibrium and pH |
| Calculating the pH of a strong acid
solution |
Calculating the pH of a weak acid solution Note: Final answer should be pH = 2.33 (2 SF) |
| Objectives 1. Correctly calculate the pH of a strong acid solution. 2. Correctly calculate the pH of a weak acid solution.` |
|
Homework Problems 24.1 As you know, strong acids completely ionize. HCl(aq) + H2O(l) →100% H3O+(aq) + Cl- (aq) This means that although a bottle might be labeled 1.00 M HCl, there is no significant concentration of HCl in the bottle. [HCl] = 0 However, knowing ionization reaction is 100/% complete and that the mole ratios are 1:1:1, we know that [ H3O+] = [Cl- ] = 1.00 M Now, using our definition of pH we obtain: pH = - log [H3O+] = - log (1.00) = 0.00 and pOH = 14.00 - pH = 14.00 (@ 25oC) Note: When performing Log calculations, only the digits AFTER the decimal point are significant. Write the dissociation reaction and calculate the pH and pOH for each of the following strong acid solutions: a. 0.010 M HNO3 b. 1.33 x 10 -5 M HCl c. 0.15 M HClO4 24.2 Determining the pH of weak acid solutions require an ICE equilibrium approach. For example, consider a bottle of acetic acid labeled 1.00 M. The bottle's concentration refers to value BEFORE equilibrium is achieved. That is, 1.00 M is the initial concentration of the acetic acid. Thus, we have the following ICE problem where we've assumed no product is initially present. CH3COOH(aq) + H2O(l) ↔ H3O+(aq) + CH3COO- (aq) Ka = 1.76 x 10-5 I 1.00 M ~ 0.00 M 0.00 M C -x +x +x E 1.00 - x x x Using the Law of Mass action and the x ~ 0 assumption lets us easily calculate [H3O+] = 4.195 x 10-3 M and pH = 2.3772 (...an acidic pH) Note: When performing Log calculations, only the digits AFTER the decimal point are significant. Thus, pH = 2.377 has three significant figures. Construct an ICE table for each of the following weak acid solutions and determine the pH of the solution. Ka values are available HERE. a. 0.500 M hydrocyanic acid b. 0.500 M benzoic acid c. 0.500 M hydrofluoric acid d. 0.500 M hypochlorous acid 24.3 All of the acids in 24.2 have the same "bottle concentration" of 0.500 M and yet they have different pH's. Compare Ka and pH for the weak acids and explain how they're related and how Ka can be used to predict the strength of a weak acid given comparable "bottle labels". Answers: Click and drag in the space below 24.1 a. pH = 2.00 (2 SF) pOH = 12.00 b. pH = 4.8761 (3 SF) pOH = 9.1238 c. pH = 0.823 (2 SF) pOH = 13.18 24.2 a. pH = 4.75 b. pH = 2.25 c. pH = 1.75 d. pH = 3.85 24.3 Smaller equilibrium constants => fewer products => lower [H3O+] => Higher (less acidic) pH |
|
.
Friday February 23, 2024 Day 34 Weak Base Equilibrium |
|
Textbook Readings 16.6: Finding the [H3O+] and pH of Strong and Weak Acid Solutions |
Course Lectures 11.3 pdf Video* Intro to Weak Base Equilibrium |
| pH of Weak Acids and Bases, Salt Solutions,
Ka, Kb, pOH Calculations |
Acid Base Equilibria, pH of Weak Bases |
| Objectives 1. Calculate Ka values from Kb values and vice versa. 2. Correctly calculate the pH of a strong base solution. 3. Correctly calculate the pH of a weak base solution.`. |
|
Homework Problems 25.1 As you know, strong bases completely dissociate in water. NaOH(s) →100% Na+(aq) + OH- (aq) This means that although a bottle might be labeled 1.00 M NaOH, there is no significant concentration of intact NaOH in the bottle. [NaOH] = 0 However, knowing dissociation is 100/% complete and that the mole ratios are 1:1:1, we know that [ Na+] = [OH- ] = 1.00 M Now, using our definition of pOH we obtain: pOH = - log [OH-] = - log (1.00) = 0.00 and pH = 14.00 - pOH = 14.00 (@ 25oC) Note: When performing Log calculations, only the digits AFTER the decimal point are significant. Write the dissociation reaction and calculate the pH and pOH for each of the following strong base solutions: a. 0.050 M KOH b. 1.50 x 10-5 M LiOH c. 2.66 x 10-4 M Ba(OH)2 25.2 As you'll read in the text book section above, the weak acid equilibrium constant Ka, is related to the weak base equilibrium constant Kb in the following way: Kw = Ka x Kb Thus, you can use an acid's Ka value to determine its conjugate base's Kb value For example: Acid: CH3COOH(aq) + H2O(l) ↔ H3O+(aq) + CH3COO- (aq) Ka = 1.76 x 10-5 Base: CH3COO- (aq) + H2O(l) ↔ OH- (aq) + CH3COOH(aq) Kb = ____?____ Where Kb = Kw /Ka = 1.00 x10-14/ 1.76 x 10-5 = 5.681 x 10-10 Examine the following weak acids, write their corresponding weak base equilibrium reactions and calculate the Kb value. a. HF Ka = 6.2 x 10-4 b. C5H5NH+ Ka = 5.90 x 10-6 c. HCO2H Ka = 1.78x 10-4 d. NH3 Ka = 1.00 x 10-35 25.3 A bottle is labeled 0.250 M NaCH3COO (sodium acetate). The pH of the solution must be determined via and ICE table: CH3COO- (aq) + H2O(l) ↔ OH- (aq) + CH3COOH(aq) Kb = 5.681 x 10-10 I 0.250 M 0.000 M 0.000 M C -x + x +x E (0.250 - x) x x Use the corresponding Law of Mass action to determine "x" , the pOH and pH of the solution. 25.4 10.55 grams of potassium fluoride is dissolved in 500.0 mL of distilled water. Useful info: For HF Ka = 7.20 x 10-4 a. Determine the initial fluoride ion concentration b. Write the corresponding weak base equlibrium reaction c. Construct an ICE table. d. Use the Law of Mass Action to determine "x" e. Determine the pOH and pH of the solution. 25.5 A 0.150 M weak base solution has a pH of 10.55 Calculate..... a. Kb for the base b. Ka for the conjugate acid Click and drag the region below for correct answers 25.1 a. KOH(s) →100% K+(aq) + OH- (aq) pOH = 1.30 and pH = 12.70 b. LiOH(s) →100% Li+(aq) + OH- (aq) pOH = 4.82 and pH = 9.18 c. Ba(OH)2(s) →100% Ba2+(aq) + 2OH-(aq) pOH =3.27 and pH = 10.72 25.2 a. F- (aq) + H2O(l) ↔ OH- (aq) + HF(aq) Kb = 1.612 x 10-11 b. C5H5N- (aq) + H2O(l) ↔ OH- (aq) + HC5H5N(aq) Kb = 1.695 x 10-9 c. HCO2- (aq) + H2O(l) ↔ OH- (aq) + H2CO2(aq) Kb = 5.618 x 10-11 d. NH3 (aq) + H2O(l) ↔ OH- (aq) + NH4+(aq) Kb = 1.000 x 1021 25.3 x = 1.191 x 10-5 M = [OH-] pOH = 4.9238 pH = 9.0761 25.4 [F-] initial = 0.363187 M F- (aq) + H2O(l) ↔ OH- (aq) + HF(aq) Kb = 1.612 x 10-11 x = 2.2459 x 10-6 pOH = 5.6486 pH = 8.3513 25.5 [H3O+] = 10-pH = 2.818 x 10-11 M [OH-] = Kw /[H3O+] = 3.548 x 10-4 M a. Kb = 8.392 x 10-7 b. Ka = 1.191 x 10-8 |
|
.
End Week 7