Monday February 26, 2024 Day 35 Percent Ionization |
|
Textbook Readings 14.3: The Relative Strengths of Acids and Bases. |
Course Lectures 11.2 pdf Video* Percent ionization |
| Objectives 1. Describe how bottle label concentration, percent ionization and pH are related 2. Explain why ionization is greater in dilute solutions than in concentrated solutions 3. Calculate the percent ionization for a weak acid solution 4. Use percent ionization to determine [H3O+], [A-] , Ka and pKa |
Lecture 11.2.
Percent Ionization. |
Homework Problems 26.1 Consider the acid ionization equilibrium reaction: HA(aq) + H2O(l) ↔ H3O+(aq) + A-(aq) Why is the forward reaction more likely to occur than the reverse reaction? 26.2 Consider two different bottles of HA solution: 1.0 M HA and 0.10 M HA a. Which of the two solutions contains greater concentrations of H3O+ & A- ? b. In which solution is it more likely that H3O+ & A- will bump into each other? c. In which solution is the reverse reaction more likely to occur? d. Percent ioniziation is a measure of how much of the original un-ionized acid is ionized. What is the effect of the reverse reaction on H3O+ & A- levels and percent ionization? e. Why do solutions with higher bottle concentrations have lower percent ionization values? 26.3 The initial concentration of an acid is 2.50 M. After an ICE problem solution, the concentrations of H3O+ & A- are both determined to be 3.55 x 10-5 M. What percent is this of the original acid concentration? 26.4 Does the ICE problem and then calculate the pH and percent ionization for 1.0 M, 0.10 M and 0.010 M HCN solutions (Ka = 4.90 x 10-10) 26.5 Based on your answers to question 26.4, how does the pH change as the initial concentration increases? 26.6 Based on your answers to question 26.4, how does the percent ionization change as the solution concentration increases? 26.7 A 0.55 M weak acid solution has a % ionization value equal to 2.44 %. Determine [H3O+] , [A-], pH and Ka for the solution. 26.8 What is the Ka and pKa value for a weak acid if a 0.35 M of the acid solution is 1.5% ionized? 26.9 Where have performed calculations like % ionization in the past? Answers: Click and drag in the space below 26.1 The forward reaction is more likely to occur because water, the solvent is so abundant. 26.2 a. The higher concentration of the 1.0 M acid solution makes product formation significant. b. Because there are more products present for the 1.0 M solution, it is more likely they H3O+ & A- will re-unite and re-form the reactants HA and H2O. c. As suggested by the answer to part "b", the reverse reaction for the 1.0 M HA solution will be more likely to occur. d. The reverse reaction, more significant for the1.0 M HA solution, significantly reduces the percent ionization for the solution overall. e. Solutions with higher initial concentrations have lower percent ionization because the reverse reaction is more significant owing to higher concentrations of products. 26.3 0.00142 % 26.4 1.0 M: pH = 4.65 and % ionization = 0.0022% 0.10 M pH = 5.15 and % ionization = 0.0070% 0.01M pH = 5.65 and % ionization = 0.022% 26.5 Higher initial concentrations → lower pH (higher [H3O+]) 26.6 Higher initial concentrations → lower % ionization. 26.7 [H3O+] = [A-] = 0.01342 M pH = 1.87 Ka = 3.27 x 10-4 26.8 Ka = 8.0 x 10-5 pKa = 4.10 26.9 Calculating % ionization is the same as the 5% rule test when performing LMA and using the x ~ 0 assumption! :) |
|
Tuesday February 27, 2024 Acid & Base Properties of Ions and Salts |
|
Textbook Readings 16.8: The Acid-Base Properties of Ions and Salts |
Course Lecture 12.2 pdf Video* Ions and their affect on pH 1 12.3 pdf Video* Ions and their affect on pH 2 |
| pH of Salts |
Salts That Contain Acidic Metal Cations and Yield Acidic Solutions |
| Objectives 1. List the strong acids and their respective anions that are pH neutral 2. List the strong bases and their respective cations that are pH neutral. 3. Identify combinations of cations and anions that are pH neutral. 4. Identify salts that contain weak acids, weak bases or both. 5. Determine the pH for salts of weak acids or bases dissolved in water |
|
Homework Problems 27.1 For each of the following, identify which anions are pH neutral and those that are not. For those that are not pH neutral, write the weak base reaction that describes their interaction with water in an aqueous environment. a. Cl- b. CH3COO- c. F- d. NO3- e. ClO4- f. CN- 27.2 For each of the following, identify which cations are pH neutral and those that are not. For the latter, write the weak acid reaction that describes the ion's interaction with water. a. Na+ b. Li+ c. NH4+ d. Al3+ e. Mg2+ f. Fe3+ 27.3 Describe, in your own words and with pictures, how it is possible for a small, highly charged metal cation to act like an acid in an aqueous environment. 27.4 10.00 grams of NaC7H5O2 are dissolved in enough water to create a 500.0 mL solution. Calculate the initial concentration of C7H5O2- and construct the weak-base ICE problem. Determine the pH of the solution. Useful information for Benzoic Acid: HC7H5O2 Ka = 6.5 x 10-5 27.5 15.00 grams of NH4Cl are dissolved in enough water to create a 750.0 mL solution. Calculate the initial concentration of NH4+ and construct the weak-acid ICE problem. Determine the pH of the solution. Useful information for NH3: Kb = 1.76 x 10-5 27.6 A solution created by dissolving solid NH4CN in water has an basic pH. Why? Useful Info: HCN Ka = 4.9 x10-10 NH3 Kb = 1.76 x 10-5 Click and drag the region below for correct answers 27.1 a. Cl- is pH neutral as it is the anion component of hydrochloric acid, a strong acid b. CH3COO- is basic as it is the conjugate base to acetic acid, a weak acid. CH3COO- + H2O(l) ↔ CH3COOH(aq) + OH-(aq) c. F- is basic as it is the conjugate base to hydrofluoric acid, a weak acid. F-(aq) + H2O(l) ↔ HF(aq) + OH-(aq) d. NO3- is pH neutral as it is the anion component of nitric acid, a strong acid. e. ClO4- is pH neutral as it is the anion component of perchloric acid, a strong acid f. CN- is basic as it is the conjugate base to hydro cyanic acid, a weak acid. CN-(aq) + H2O(l) ↔ HCN(aq) + OH-(aq) 27.2 a. Na+: pH neutral This cation is associated with NaOH, a strong base. b. Li+ : pH neutral. This cation is associated with LiOH, a strong base. c. NH4+ : Acidic pH This is the conjugate acid of a weak base (NH3). NH4+ + H2O(l) ↔ NH3 + H3O+(aq) d. Al3+ : Acidic pH This is the conjugate acid of a weak base (Al(OH)3)). Note that Al3+ , when in a water environment, is surrounded by H2O molecules. The positive charge is responsible for shifting electron density out of the O-H bonds... ...weakening them and creating an "acidic" situation. Al(H2O)63+ + H2O(l) ↔ Al(H2O)5OH2+ + H3O+(aq) e. Ca+2 : pH neutral. This cation is associated with Ca(OH)2, a strong base. f. Fe3+ : Acidic pH This is the conjugate acid of a weak base (Fe(OH)3)). Note that Fe3+ when in a water environment, is surrounded by H2O molecules. The positive charge is responsible for shifting electron density out of the O-H bonds... ...weakening them and creating an "acidic" situation. Fe(H2O)63+ + H2O(l) ↔ Fe(H2O)5OH2+ + H3O+(aq) 27.3 Highly charged cations attract the negative (oxygen) ends of water molecules. There is room for six water molecules to gather typically around a metal cation. The metal ion's positive charge shifts electron charge density out of O-H bonds weakening them and making it possible for H+ ions to leave (acidic behaviour). 27.4 pH = 8.66 27.5 pH = 4.84 27.6 In this case, there are two competing equilibria: NH4+ + H2O(l) ↔ NH3 + H3O+(aq) Ka = 5.68 x 10-10 CN-(aq) + H2O(l) ↔ HCN(aq) + OH-(aq) Kb = 2.04 x 10-5 Of the two reactions, the second produces more product than the first (based on a comparison of the two equilibrium constants). Thus there is more OH- in solution than H3O+ and the solution is basic. |
|
.
Wednesday February 28, 2024 Day 37 Acid Strength and Molecular Structure |
|
Textbook Readings 16.10: Acid Strength and Molecular Structure 16.11: Lewis Acids and Bases |
Course Lectures 13.1 pdf Video* Acid Strength: Bond Length and Bond Polarity 13.2 pdf Video* Oxoacids and the Lewis Model |
| Objectives 1. Describe important differences between strong and weak acids 2. Predict relative acid strengths based upon the strength of the bond attaching the hydrogen to the acidic molecule 3. Predict relative acid strengths based upon the LENGTH of the bond attaching the hydrogen to the acidic molecule |
Molecular Structure and Acid Strength |
4. Predict the effect of molecular and the presence of electron withdrawing groups on bond integrity and acid strength. 5. Describe what's known as the "Leveling Effect" of water and how it applies to acid strength. |
|
Homework Problems 28.1 Examine the two acide below and decide which is most acidic and why. (Source) 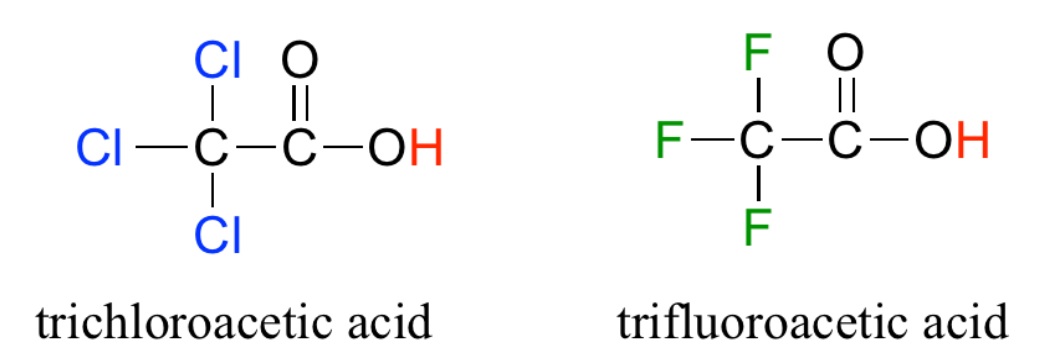 28.2 Examine the two acids below and decide which is most acidic and why.(Source) 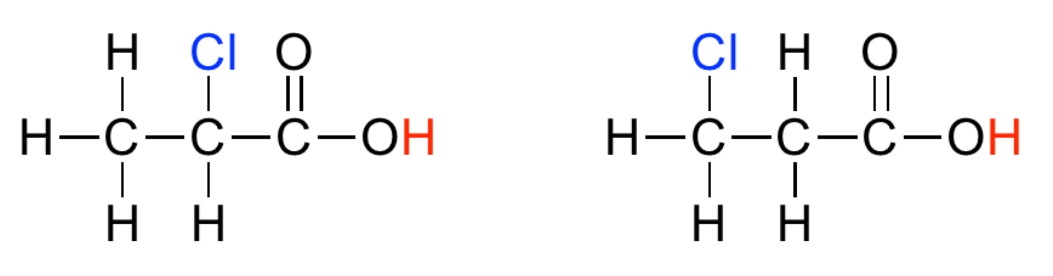 28.3 Examine the four acids below and decide which is the most acidic and why.(Source) 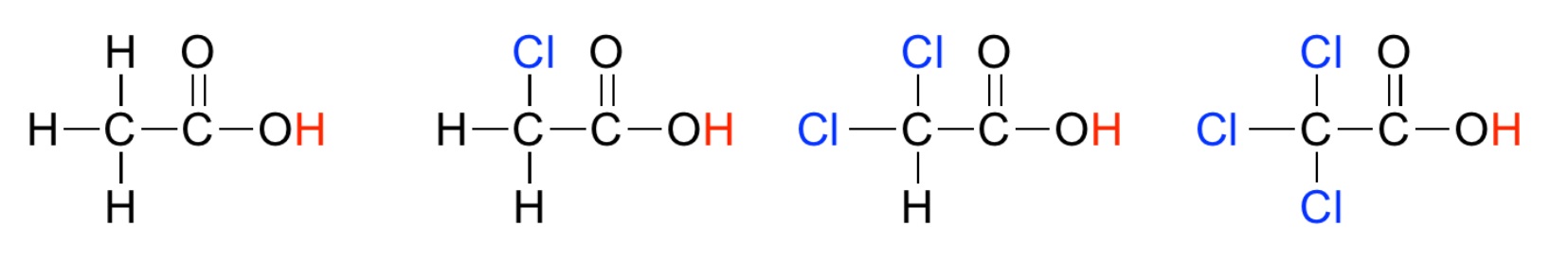 28.4 For each of the following acids, calculate Ka and then put them in order of increasing acid strength: HI (pKa = -10) HCl (pKa = -7) HBr (pKa = -9) 28.5 In water, all of the acids in question 28.4 completely ionize and appear equally strong. The "Leveling Effect" of water explains this puzzling result. Describe, in your own words, how water makes it possible for all of the acids in 28.4 to appear equally strong. Click and drag the region below for correct answers 28.1 The right hand structure is more acidic than the left hand structure because fluorine is more electronegative and shifts more electron density out of the O-H bond. This weakens the O-H bond and the hydrogen atom is more loosely attached; the identifying feature of a STRONG acid. 28.2 The left hand structure is more acidic. In this case, the electronegative chlorine atom is closer to the O-H bond making it possible to more effectively shift electrons out of the OH bond. Thus, the acidic hydrogen atom is more loosely held and the acid is STRONG. 28.3 The far right hand structure is most acidic where three chlorine atoms work together to shift electron density from the O-H bond making it weaker and the acid stronger. 28.4 Weak... HCl (Ka = 1 x 107) HBr (Ka = 1 x 109) HI (Ka = 1 x 1010) ...Strong 28.5 A strong acid is observed to ionize completely and this is dependent to a large degree on the strength of the bond holding the acidic hydrogen (Weak bond → Strong acid) However, the H2O is a strong base and more than willing to help even weaker acid, HCl, completely ionize. Thus, water "levels the playing field" and makes all three acids appear equally strong. Dissolved in a liquid that wasn't such a strong base, the three acids would ionize to different degrees and would exhibit different acid strengths. |
|
Thursday February 29, 2024 Day 38 Strong Acid/Base Titrations: pH @ Equivalence (End) Point & Polyprotic Acids |
|
Textbook Reading 13.5 Acid/Base Titration 16.9: Polyprotic Acids |
Course Lectures 3.4 pdf Video* Solutions: Molarity and Titrations |
| Objectives 1. Recognize strong acids and bases by their chemical formulae 2. Write balanced neutralization reactions for strong acids and bases. 3. Perform neutralization calculations where the concentrations of all species at the equivalence point are determined. 4. Determine the pH of the solution at different times throughout the titration. 6. Determine the pH of the solution at the equivalence point of the titration. |
Strong Acid-Strong Base Titration Problems with pH Calculations |
Homework Problems 29.1 100.0 ml of a 0.75 M HCl solution is titrated with 0.30 M NaOH. a. What is the chemical equation that describes this neutralization reaction? b. When 50.0 mL of NaOH have been added.... i. How many moles of the HCl remain unreacted? ii. What is the total volume of the solution at this point? iii. What is the [H3O+] at this point? iv. What are the pH and pOH at this point? c. When 250.0 mL of NaOH have been added.... i. How many moles of the HCl remain unreacted? ii. What is the total volume of the solution at this point? iii. What is the [H3O+] at this point? iv. What are the pH and pOH at this point? d. When 300.0 mL of NaOH have been added.... i. How many moles of the NaOH remain unreacted? i. What is the total volume of the solution at this point? iii. What is the [OH-] at this point? iv. What are the pOH and pH at this point? 29.2 45.00 mL of a 0.250 M H2SO4 solution is titrated with 0.100 M NaOH. a. What is the chemical equation that describes this neutralization reaction? b. What is the volume in mL of NaOH required to reach the equivalence point? c. At the equivalence point, what are the sodium and sulfate ion concentrations? d. At the equivalence point, what are the pH and pOH e. How has the significant concentration of sodium and sulfate ions affected the pH? 29.3 200.0 mL Ca(OH)2 are titrated with 0.0450 M HNO3. a. What is the chemical equation that describes this neutralization reaction? b. If the endpoint has been reached after the addition of 45.3 mL of HNO3, what is the concentration of the Ca(OH)2? What is the pH at this point? c. If the chemist over-shoots the equivalence point of the titration by 0.15 mL of HNO3, what is the pH of the solution? 29.4 Sulfuric acid, H2SO4 is a di-protic acid that has 2 acidic hydrogen atoms. Ionization occurs in two stages: 1. H2SO4(aq) + H2O(l) →100% HSO4- (aq) + H3O+(aq) Ka1 > 100 2. HSO4- (aq) + H2O(l) →100% SO4-2 (aq) + H3O+(aq) Ka2 = 1.2 x 10-2 Note that the first ionization would be considered a "completion reaction" since it has a very large equilibrium constant. It is therefore safe to assume all of the initial H2SO4 is converted to HSO4- and H3O+. Set up an ICE table for the second reaction and determine the equilibrium concentrations of HSO4- , SO4-2 and H3O+ for a bottle of 1.00 M sulfuric acid. 29.5 How is it possible to completely neutralize sulfuric acid when the second ionization reaction produces only limited numbers of hydronium ions? Click and drag the region below for correct answers 29.1 a. HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq) b. i 75 mmolHCl - 15 mmolNaOH = 60. mmolHCl (Note the use of "milli-moles") ii. 50.0 mL + 100.0 mLHCl = 150.0 mL iii. 60. mmolHCl / 150.0 mLtot = 0.40 MHCl = [H3O+] iv. pH = 0.3979 pOH = 13.6020 Note: the pH of the solution is determined by the excess HCl. Although the autoionization of water still contributes to the solution's pH, it is a minimal effect. c. i. 75 mmolHCl - 75 mmolNaOH = 0 mmolHCl ii. 250.0 mL + 100.0 mL = 350.0 mL iii. Enough NaOH has been added to the HCl solution to completely neutralize it. We're at the equivalence point in the titration where molHCl = molNaOH [H3O+] & [OH-] are determined entirely by the autoionization of water: H2O(l) + H2O(l) → H3O+(aq) + OH-(aq) Kw = 1.00 x 10-14 @ 25oC So, [H3O+] = [OH-] = 1.00 x 10-7 M iv. pH = pOH = 7 d. i. 90. mmolNaOH - 75 mmolHCl = 15 mmolNaOH ii. 300.0 mL + 100.0 mL = 400.0 mL iii. [OH-] = 0.0375 M iv. pOH = 1.4259 pH = 12.574 Note: the pH of the solution is determined by the excess NaOH. Although the autoionization of water still contributes to the solution's pH, it is a minimal effect. 29.2 a. H2SO4(aq) + 2 NaOH(aq) → 2 H2O(l) + Na2SO4(aq) b. 225.0 mL of NaOH required to reach the equivalence point. c. [Na+] = 0.0833 M [SO42-] = 0.416 M d. We're at the equivalence point of a strong acid/strong base titration. pH is determined by the autoionization of water: pH = pOH = 7.00 @ 25oC e. Sodium is the cation of the strong base, NaOH. Chloride is the anion of a strong acid, HCl. Ions like these do not affect the pH of a solution. 29.3 a. Ca(OH)2 + 2 HNO3 → 2 H2O(l) + Ca(NO3)2(aq) b. 5.096 x 10-3 MCa(OH)2 pH = 7.00 (Strong acid/strong base titration) c. pH = 4.5606 (Amazing how such a small amount of excess HNO3 changes the pH) 29.4 HSO4- (aq) + H2O(l) →100% SO4-2 (aq) + H3O+(aq) Ka2 = 1.2 x 10-2 Initial 1.00M ~ 0.00 M 1.00 M Note: this will require the quadratic equation as assumptions are not valid. [HSO4-]eq = 0.988 M [SO4-2]eq = 0.0112 M and [H3O+]eq = 1.01 M 29.5 Although a significant amount of HSO4- remains intact and un-ionized, neutralization converts H3O+ into water. As the [H3O+ ] drops, the second reaction shifts right, converting HSO4- into replacement H3O+ . This continues until the second reaction can no longer shift right and there is no HSO4- left. |
|
Friday March 1, 2024 Day 39 Weak Acid/Strong Base Titration |
|
Textbook Readings Titration of a Weak Acid with a Strong Base |
Course Lectures 15.01 pdf Video* Intro to Weak Acid titration 15.02 pdf Video* Buffer Region pH determination |
| Introduction to Weak Acid titrations |
Buffer Region pH determination |
| Objectives 1. Calculate initial moles of weak acid and strong base. 2. Determine the remaining moles of weak acid after neutralization takes place. 3. Determine the moles of conjugate base produced during the neutralization reaction. 4. Recalculate concentrations of weak acid and conjugate base using the new total solution volume. 5. Configure an ICE problem solution using the new concentrations of weak acid and conjugate base. 6. Calculate the pH and pOH of the solution. |
|
Homework Problems 30.1 A beaker contains 50.00 mL of 0.100 M CH3COOH a. How many milli-moles (mmol) of acetic acid are initially present? b. 6.00 mL of 0.100 M NaOH added to the beaker and neutralized by the acetic acid. How many mmols of NaOH have been added? c. Acetic acid neutralizes the NaOH via the following neutralization reaction: CH3COOH(aq) + NaOH(aq) → H2O(l) + NaCH3COO-(aq) How many mmols of CH3COOH(aq) are left after the NaOH has been added? How many mmols of CH3COO-(aq) are produced? Note that Na+ is a spectator ion in this problem that doesn't affect pH. d. Use the total solution volume and mole amounts from part "c" to determine [CH3COOH] and [CH3COO-] at this point in the titration. e. The pH of the solution at this point in the titration is determined by the acetic acid/ acetate equilibrium: CH3COOH(aq) + H2O(l) ↔ H3O+(aq) + CH3COO-(aq) Ka = 1.76 x 10-5 I __________ 0.00 M _________ C __________ __________ _________ E __________ __________ _________ Using the concentrations you calculated in part "d" as the initial concentrations, determine [H3O+] and the pH for this solution. 30.2 For the solution above, determine the pH after the following NaOH additions: a. 10.0 mL NaOH b. 25.0 mL NaOH c. 40.0 mL NaOH d. 45.0 mL NaOH 30.3 The "half (½) equivalence point" is defined at the point where the concentrations of the weak acid (CH3COOH) and conjugate base (CH3COO-) concentrations are equal. a. Which of the pH's in problem 30.2 corresponded to the ½ equivalence point? b. At the ½ equivalence point, pH = log(Ka). Verify that this is true. 30.4 Quite a lot of NaOH was added to the acetic acid. Create a simple graph of pH vs mLNaOH using the five pH values you determined above. The pH axis should span the pH = 0 to pH = 14 range. Label the data on the graph as "Buffering Region" Describe in words the pH changes observed as you added NaOH. Click and drag the region below for correct answers 30.1 a. 5.00mmoles b. 0.60 mmoles c. 4.40 mmol CH3COOH left after neutralization. 0.60 moles of CH3COO- produced. d. [CH3COOH] = 0.078571 M [CH3COO-] = 0.010714 M e. [H3O+] = 1.2907 x 10-4 M pH = 3.889 30.2 a. pH = 4.15 b. pH = 4.75 c. pH = 5.36 d. pH = 5.71 30.3 a. The ½ equivalence point is reached when 25 mL of NaOH are added to the acetic acid solution. b. At the ½ equivalence point pH = -logKa = - log(1.76 x 10-5) = 4.75 :) 30.4 The pH is slowly increasing. Graph click here. |
|
.
End Week 8